File
advertisement

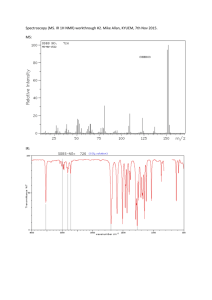
Cash 1 The Classification of an Unknown Substance Using Wet Tests and NMR, IR, and Mass Spectrometry Abstract In this laboratory experiment a chemical of unknown identity was tested for physical/chemical properties pertaining to specific functional groups. After several tests, the functional group present in the molecule was determined, and the configuration of the molecule was deduced using H-NMR, C-NMR, IR spectroscopy, and mass spectrometry. The unknown was a white solid at room temperature with a melting point of 76.0-77.0 Celsius. There was almost no odor emitted from the compound. Important IR peaks were located at approximately 3200-3600 cm-1, 1720 cm-1, and 1400-1500 cm-1. C-NMR showed peaks at 178ppm, 43ppm, and multiple peaks in the 125-130ppm region. H-NMR had only 3 peaks: one singlet located at 9.7ppm, a multiplet at 7.3ppm, and a singlet at 3.6ppm. Perhaps most importantly, the mass spectrum’s parent peak was located at 136m/z, and the base peak was located at 91m/z. Cash 2 Introduction The purpose of the experiment was to determine the composition of an unknown substance with limited quantity. This analytical approach of determining the structure of a compound resembles what one would do in a research environment. Certain preliminary classifications are necessary and very helpful in classifying a compound. Those include: the color, odor, state at room temperature, density, and pH. After these classifications, the first test that should be done to a solid compound should be to determine what it is soluble in. Figure 1 shows the general solubility rules for compounds containing various substituents. Essentially, the IR spectrum can initially be used to show what substituents you may have, then the wet tests and the other spectra can support the initial hypothesis. One of the most helpful wet tests is the solubility test, as it can quickly rule out possible functional groups and narrow it down to only one or two. After the solubility test is completed, chemical classification tests can be conducted based on what functional group is present (shown from the solubility test). Once the tests are completed, and the functional group identified, the remaining spectra can be used for the configuration of the entire molecule. One of the most important spectrums to analyze when determining an unknown is the mass spectrum. With it, the weight of the molecule and the chemical formula can be found. In addition to the mass spec., Nuclear Magnetic Resonance (NMR) can be used to determine the amount of Hydrogen (H) and Carbon (C) environments. If any electronegative substituent groups are located Cash 3 on the molecule, we should expect to see peaks shifted downfield in the C-NMR and H-NMR. This is because the electronegative groups will de-shield any carbon and hydrogen environments that are near it. Insert picture of solubility of compound Figure 1. Summary of solubility of various functional groups. In bold represents route of unknown. Procedure Preliminary classification tests were completed and recorded (i.e. odor, color, etc.). Next, the solubility of the compound was tested in water, sodium hydroxide, and hydrochloric acid. Using the information gathered from the solubility tests, and referring to Figure 1, we determined what substituent was most likely in our compound, and conducted chemical classification tests for said substituent. Finally, we used the four spectrums (MS, C-NMR, H-NMR, IR) to determine the structure of our molecule. Results RCOOH(S) + NaHCO3 (l) RCOO- Na+(aq) + H2CO3 H2CO3 CO2(g) + H2O(aq) Cash 4 Figure 2: Structure of unknown molecule. Wet Tests Ignition Test Ph Test Melting Point Halogen Test Water Sodium Hydroxide Sodium Bicarbonate Positive Approximately 3-4 76.7-78.1 Negative Not Soluble Soluble Soluble+gas Table 1: Wet test results Please see attachment for spectrums. Discussion and Conclusion The parent peak (M) located at 136m/z shows that our molecule has a mass of 136amu’s. The base peak (M-45) located at 91m/z refers to the formation of a tropylium ion, after the loss of the carboxylic acid. Turning to the IR, a broad peak located around 3600-3200 cm-1 and a sharp peak at 1720 cm-1 are a good indication of a carboxylic acid. In the H-NMR a singlet located at 9.7ppm is yet another indicator of a carboxylic acid. This, along with the other evidence from the wet test and the C-NMR peak at 178ppm, proves there is a carboxylic acid. Aromatic C-H bending absorbance patterns are located in the IR spectrum at 1035 cm-1 and 1080 cm-1. In addition C-C stretches in the aromatic ring are located at around 1450 cm-1-1500 cm-1. No further evidence in the IR shows aromatic absorption patterns, because they are covered up by the carboxylic acid peaks. There were multiple peaks located at 125-130ppm in the C-NMR, further Cash 5 suggesting an aromatic ring. In addition, the positive result from the ignition test proved that there was some degree of unsaturation in the structure. Finally, the multiplet located at 7.3ppm in the H-NMR is proof of an aromatic α-carbon, therefore, all of the evidence suggests there must be an aromatic ring with a carboxylic acid substituent. Considering the mass spectrum and the formation of the tropylium cation after the loss of the carboxylic acid, it was determined that there must be a methylene substituent located in between the carboxylic acid and the benzene ring. The evidence from the H-NMR supports this, as a peak at 3.6ppm with an integration value of 2 was present. The C-NMR also shows a peak at 41ppm indicating a βcarbon region not on the benzene ring or corresponding to the carbon in the carboxylic acid. Combining all of the information gathered from the wet tests and the mass spectrum, IR, and NMR, we deduced that our unknown was phenylacetic acid. The experimental melting point and the theoretical melting point of phenylacetic acid were identical, and the molecular weight of phenylacetic acid is, in fact, 136amu’s, as our mass spec showed. Therefore, the unknown substance is phenylacetic acid. Cash 6