Ch. 3 – Test Study Guide
advertisement

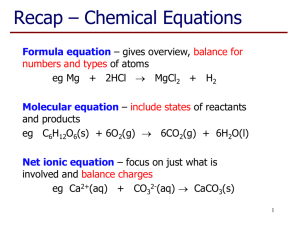
CP Chemistry Chapter 3 – Study Guide Test Date: Thursday, October 16, 2014 Use the following to study: Textbook – Chapter 3 Ch. 3 PPT notes Ch. 3 packet Chapter Three History of Atomic Theory o Democritus o Dalton Dalton’s Atomic Theory What was incorrect about Dalton’s theory? o JJ Thompson Cathode Ray Tube Experiment and results (what is he credited with discovering?) Plum Pudding Model of the atom (be able to describe) o Robert Millikan Oil Drop Experiment and results o Earnest Rutherford Gold Foil Experiment What did Rutherford expect to see in his experiment based on the Plum Pudding Model? What did he actually see? What conclusion did he come to as a result of this? Nuclear Model of the atom o James Chadwick Discovered the neutron in 1932 Three Basic Laws o Law of Conservation of Mass o Law of Definite Proportions o Law of Multiple Proportions Structure of the Atom o Nucleus – what is inside of it? What is its mass compared to the whole atom? o Subatomic particles – protons, electrons, neutrons What are their charges? Where are they found inside the atom? Which two have essentially the same mass? Which one is so small its mass is ignored? o The majority of the atom is_______________________________________. o Nuclear forces – what are they? What are the responsible for? o Atomic Radius – what does it measure? What units is it measured in? Determining Subatomic Particles in an Atom o Atomic Number = # of protons (and # of electrons in a neutral atom) o Mass Number = # protons + # neutrons Isotopes o Definition o What is different among isotopes (2 things)? What must always be the same? o know the difference between percent abundance & relative abundance o calculations of average atomic mass and its units! Isotope Notation o Nuclear Notation (example below) o (know what the 8 & 16 represent) Hyphenated Notation Oxygen-16 (know what the 16 represents) The Mole & Molar Conversions o What does the mole represent? o Avogadro’s # will be given to you on the day of the test (6.022 x 1023 atoms/mol) o Molar Mass – definition, units, determining molar mass of elements o Molar Conversions (must use dimensional analysis!!!) Grams Moles (and reverse) Atoms Moles (and reverse) Grams Atoms (and reverse)… these ones are the two step problems o On the test you will only be given the following: You MUST use DIMENSIONAL ANALYSIS to solve these problems… the map below is only a guide to help you think through the problem The Mole Map: Grams Moles Atoms