Lesson 3 - WordPress.com
advertisement

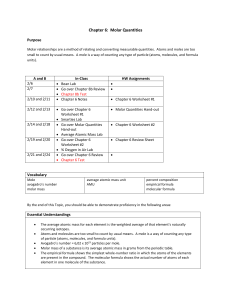
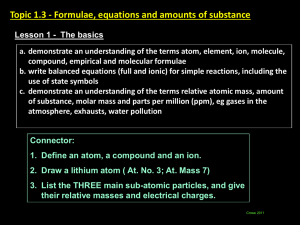
Lesson 3 Mass Objective: To explore different types of mass of elements, and be introduced to the measurement of moles. Relative Isotopic Mass This is the mass of an atom of an Isotope compared with 1/12 of the mass of an atom of Carbon-12. Example: Oxygen-16 has a relative Isotopic mass of 16.0, Sodium-23 has a relative Isotopic mass of 23.0. Relative Atomic Mass (Ar) The relative atomic mass is the weighted mean mass of an atom of an element, this is calculated using the different masses and relative abundances of all the Isotopes of a particular element. For example: 35 Cl 17 37 Cl 17 If I am told that 75% of Chlorine atoms have an atomic mass of 35, and 25% have an atomic mass of 37, I can calculate the relative atomic mass. (35x75) + (37x25) ________________ = 35.5 100 35.5 is therefore the relative atomic mass of Chlorine, an average taken using the types and amounts of other Isotopes of the element. Another example: 24 Mg 12 25 12 78.6% Mg 10.1% 26 Mg 12 11.3% (24 x 78.6) + (25 x 10.1) + (26 x 11.3) By Ruth N. www.ruthlearns.wordpress.com < abundance of each Isotope of the element magnesium. ________________________________ = 24.3 100 As you can see Mg 24 is the most common / abundant Isotope, so the final average is nearest to 24. Relative atomic mass allows for the most accurate average mass of a particular element. What is the difference between Relative Isotopic Mass and Relative Atomic Mass? This is simple; Relative Atomic Mass takes into account ALL the Isotopes of a particular element (as demonstrated above), whereas Relative Isotopic Mass means the mass of just ONE Isotope of a particular element. Example: Chlorine-35 has relative ISOTOPIC mass of 35. Chlorine-37 has a relative isotopic mass of 37. But if we want the relative ATOMIC mass, we must add the isotopes, multiply by abundance, and divide by one hundred to find an average mass (this exact question was covered above). Relative Formula Mass Also known as relative molecular mass (Mr). Relative Formula Mass is the weighted mean mass of a molecule (compared with 1/12 of the mass of an atom of Carbon-12). So, where Relative Atomic Mass dealt with an atom of a whole element, Relative Formula Mass deals with the mass of a molecule (a molecule is made up of two or more chemically bonded atoms). Many elements and compounds are made up of simple molecules like N2, O2 or CO2 Compounds with giant structures do not exist as molecules, for example: Ionic compound NaCl, or covalent compound SiO2, so ‘relative formula mass’ is seen as more accurate than saying molecular mass. Remember: Atoms, Molecules, and Compounds; “A molecule is formed when two or more atoms join together chemically. A compound is a molecule that contains at least two different elements. All compounds are molecules but not all molecules are compounds. Molecular hydrogen (H2), molecular oxygen (O2) and molecular nitrogen (N2) are not compounds because each is composed of a single element. Water (H2O), carbon dioxide (CO2) and methane (CH4) are compounds because each is made from more than one element. The smallest bit of each of these substances would be referred to as a molecule. For example, a single molecule of molecular hydrogen is made from two atoms of hydrogen while a single molecule of water is made from two atoms of hydrogen and one atom of oxygen.” http://education.jlab.org/qa/compound.html By Ruth N. www.ruthlearns.wordpress.com To calculate the relative formula mass (Mr) of a substance all you have to do is add up the relative atomic masses of all the elements. H2O Water there fore has a relative formula mass of 18. Hydrogen has an atomic mass of 1, there are 2 atoms of Hydrogen in water so multiply by 2. Oxygen has an atomic mass of 16, so 16 + 2 = 18. What is the relative formula mass of K2CO3 ? K2 – 1 atom of Potassium has atomic mass of 39.1, but there are 2 atoms here so x2 is 78.2 C - see on the periodic table, atomic mass 12 O – We already established has atomic mass 16, but there are 3 atoms of it here so x3 is 48 78.2 + 12 + 48 = 138.2 Introduction to the mole The mole is a measure of the amount of a substance. 1 mole of an element is equal to that particular element’s relative formula mass or relative atomic mass. (1mol = RFM and RAM) Example: 1 mole of oxygen has a molar mass/relative formula mass of 16gmol-1 (the unit gmol-1 literally means grams per mole, so there is 16g per mole of oxygen, 1 mole of oxygen has a mass of 16g) As we know 16 is oxygen’s relative formula mass, and its relative atomic mass listed on the periodic table. If we have 2 moles of oxygen, then its ‘molar mass’ (same as relative formula mass) would be 32gmol-1 But what if we are given the weight of an element in grams and told to calculate the amount of moles? If I have 50g of Oxygen, all I need to do is divide that by Oxygen’s molar mass/relative formula mass (16gmol-1) and I get 3.125 moles. (Test this by multiplying 3.125mol by 16gmol-1). This is all illustrated in the calculation formula triangle below: By Ruth N. www.ruthlearns.wordpress.com 1 mole of a substance contains 6.02x1023 particles/atoms, this number is known as Avogadro’s number and you can’t escape it in chemistry. A book I highly recommend is Calculations in AS / A Level Chemistry by Jim Clark, goes into detail with moles but keeps it simple and fun, with plenty of practice questions. The book is not just about moles, it’s everything, and has been essential. You can get a second hand version pretty cheap here: http://www.amazon.co.uk/Calculations-AS-A-LevelChemistry/dp/0582411270/ref=sr_1_cc_1?s=aps&ie=UTF8&qid=1335368574& sr=1-1-catcorr The bottom row multiplies, the top row divides. So… moles x molar mass = mass molar mass x moles = mass (same thing) mass ÷ moles = molar mass mass ÷ molar mass = moles It’s very straightforward, so the above may seem patronising but it’s just in case anyone finds triangles tricky. The other formula triangles are below. By Ruth N. www.ruthlearns.wordpress.com By Ruth N. www.ruthlearns.wordpress.com