Chemical Ideas, Unit EL 1
advertisement
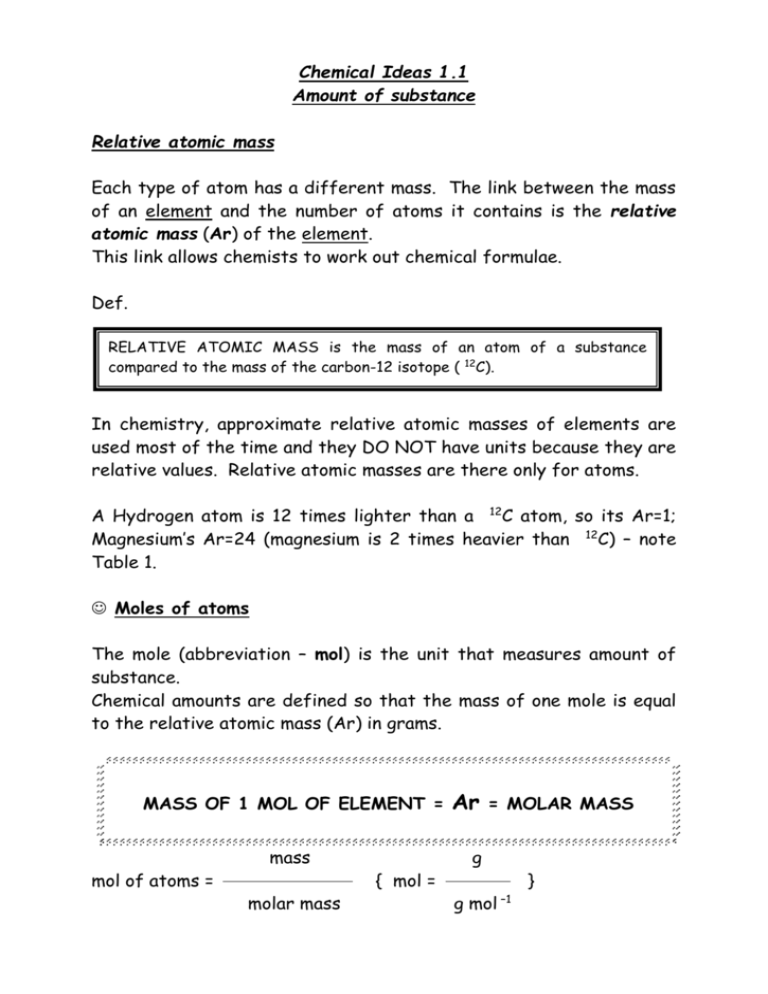
Chemical Ideas 1.1
Amount of substance
Relative atomic mass
Each type of atom has a different mass. The link between the mass
of an element and the number of atoms it contains is the relative
atomic mass (Ar) of the element.
This link allows chemists to work out chemical formulae.
Def.
RELATIVE ATOMIC MASS is the mass of an atom of a substance
compared to the mass of the carbon-12 isotope ( 12C).
In chemistry, approximate relative atomic masses of elements are
used most of the time and they DO NOT have units because they are
relative values. Relative atomic masses are there only for atoms.
A Hydrogen atom is 12 times lighter than a 12C atom, so its Ar=1;
Magnesium’s Ar=24 (magnesium is 2 times heavier than 12C) – note
Table 1.
Moles of atoms
The mole (abbreviation – mol) is the unit that measures amount of
substance.
Chemical amounts are defined so that the mass of one mole is equal
to the relative atomic mass (Ar) in grams.
MASS OF 1 MOL OF ELEMENT =
mol of atoms =
mass
molar mass
{ mol =
Ar
= MOLAR MASS
g
g mol –1
}
Relative formula mass and Relative molecular mass
Molecules are different from the single atoms. They consist of few
atoms chemically joined together unless they are diatomic elements
(H2 , N2 , O2 , F2 and the rest of the halogens) which only have one
type of atoms chemically joined together.
Chemists use relative formula masses to compare ionic substances
and they have NO UNITS.
For substances where molecules are formed by covalent bonding
chemists use relative molecular masses to compare them and they
DO NOT HAVE UNITS.
Both relative formula mass and relative molecular mass are given the
symbol Mr.
Def.
RELATIVE FORMULA MASS is the mass of 1 molecule of a compound
relative to the mass of a 12C atom.
To find the value of Mr you need to add the individual Ars of the
elements together.
Formula units
Formula units can be single atoms (all metals as elements), molecules
(diatomic elements; covalent compounds) or groups of ions (in ionic
compounds)
Moles of formula units
The relative formula mass (Mr) in grams is equal to the molar mass
of a compound:
methane’s Mr=16
molar mass is 16g
mass (g) = amount of moles of formula units
molar mass
The Avogadro constant
The number of formula units (atoms; molecules; ions; electrons) in
one mole of a substance is a CONSTANT. It is called the Avogadro
constant ( symbol L) and its value is 6.02 x 1023 formula units mol-1.
Chemical formulae
We use moles to find out the formula of a compound. Chemists use
two types of the formulae: empirical and molecular.
The empirical formula of a substance is the simplest formula. It
tells you the ratio of the numbers of different types of atom in the
substance.
The molecular formula tells you the actual numbers of different
types of atom.
What do chemical formulae tell you?
A number of moles of certain elements in a compound.
(NH4)3PO4
3 moles of nitrogen
12 moles of hydrogen
4 moles of oxygen
1 mole of phosphorus
How to work out the empirical formula and the molecular
formula?







