Revised_Measuring Moles Activity
advertisement

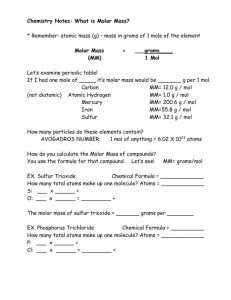
UNIT 1 – C6: Measuring Moles Activity Name: ____________________ Physical Science Date: ____________________ Objective: Determine the amount of mass and volume for ONE MOLE of different substances. Material Formula Magnesium Hydroxide Copper Water Salt Sugar Mg(OH)2 Cu H2O NaCl C12H22O11 # of different types of atoms 3 # of Atoms per molecule 5 One Mole of a substance is 6.022 x 1023 particles (either atoms or molecules) of that substance. This is similar to other groups of items, like a dozen doughnuts, a pair of shoes, even a cup of sugar for a recipe is similar. The Mole is useful in chemistry because it represents a number of atoms, molecules, or formula units large enough to be conveniently weighed or measured in the laboratory. Example: if an egg has a mass of 50 grams, a dozen eggs is just 12 eggs added together, so a dozen eggs has a total mass of 600 grams. The molar mass of any particular atom is shown on the periodic table as the average atomic mass in grams per mole! To calculate the molar mass of a compound you need to take atom inventory and add the masses of the individual atoms. 1. Find the mass of each type of atom 2. Add together the total mass of each type of atom in the molecule For example the molar mass of Mg(OH)2 is 1 atom of Magnesium (24.3 g/mol) + 2 atoms of oxygen ( 2 X 16 g/mol) + 2 atoms of hydrogen ( 2 X 1 g/mol) = 24.3+ 32+ 2= 58.3 g/mol. The molar mass is the amount of grams in mole of the compound. 1 mole of Mg(OH)2=58.3g Calculate the molar mass for Sugar if the formula is C6H12O6, NaCl, H2O, Cu. Material Formula Copper Water Salt Sugar Cu H2O NaCl C12H22O11 Molar mass 1. Compare the mass of a mole of each sample and the formula mass. What pattern do you observe? 2. What do you need to do to predict the mass of a mole of any substance if the formula is known? (List the steps) 3. Predict the mass of one mole of each of the following: a. Ca(OH)2 b. H2SO4 c. Al(NO3)3 4. Find the mass of 3 moles of each of the substances in # 3. a. Ca(OH)2 b. H2SO4 c. Al(NO3)3 5. Write the steps for calculating the mass of a given number of moles. 1. 2. 6. Use the above rule to find the mass of each of the following: a. One mole of CO b. Five moles of CO