File
advertisement

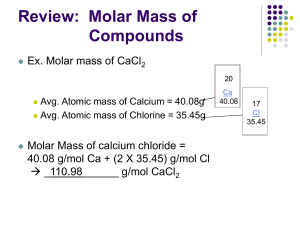
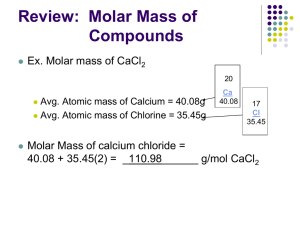
Topic 1.3 - Formulae, equations and amounts of substance Lesson 1 - The basics a. demonstrate an understanding of the terms atom, element, ion, molecule, compound, empirical and molecular formulae b. write balanced equations (full and ionic) for simple reactions, including the use of state symbols c. demonstrate an understanding of the terms relative atomic mass, amount of substance, molar mass and parts per million (ppm), eg gases in the atmosphere, exhausts, water pollution Connector: 1. Define an atom, a compound and an ion. 2. Draw a lithium atom ( At. No. 3; At. Mass 7) 3. List the THREE main sub-atomic particles, and give their relative masses and electrical charges. Crowe 2011 An atom is the smallest, electrically neutral, particle of an element that can take part in a chemical change. A molecule is the smallest, electrically neutral, particle of an element or compound that can exist on its own. An ion is an atom, or group of atoms, which carries an electric charge. Lithium Atom A Lithium has 3 protons and 4 neutrons inside the nucleus with 3 electrons orbiting around the nucleus. Write the word equation and then the balanced equation, including state symbols for the following reactions: 1. Hydrogen and oxygen combining to form water. 2. Calcium carbonate decomposing into calcium oxide and carbon dioxide. 3. Hydrochloric acid reacting with magnesium to produce hydrogen gas and a solution of magnesium chloride. 4. Sulphuric acid being neutralised by sodium hydroxide to give a solution of sodium sulphate. 5. Zinc oxide reacting with sulphuric acid to give a solution of zinc sulphate. 6. Aluminium combining with oxygen to form its oxide. 7. Methane under going complete combustion. Relative atomic mass & molar mass Explain these terms. RAMS & MOLES Relative atomic mass The definition of Relative Atomic Mass (Ar): The mass of a single atom on a scale on which the mass of an atom of carbon—12 has a mass of 12 atomic mass units. 12 hydrogen atoms have the same mass as 1 carbon atom The relative atomic mass does not have units. Relative Atomic Mass of elements The atoms of each element have a different mass. Carbon is given a relative atomic mass (RAM) of 12. The RAM of other atoms compares them with carbon. Eg. Hydrogen has a mass of only one twelfth that of carbon and so has a RAM of 1. Below are the RAMs of some other elements. Element Symbol Times as heavy as carbon R.A.M Helium He one third 4 Beryllium Be three quarters 9 Molybdenum Mo Eight 96 Krypton Kr Seven 84 Oxygen O One and one third 16 Silver Ag Nine 108 Calcium Ca Three and one third 40 Molar mass or Moles The mole is the formula mass in g’s of an atom, molecule, or compound. Example: Water H2O = (2xH)+(O) = (2x1)+16 = 18 So water has a molar mass of 18g or 1 mole of water weighs 18g Formula Mass Calculate the formula mass of the compounds below. (N=14; H=1; Na=23; O=16; Mg=24; Ca=40) Substance Formula Formula Mass Ammonia NH3 14 + (3x1)=17 Sodium oxide Na2O (2x23) + 16 =62 Magnesium hydroxide Mg(OH)2 24+ 2(16+1)=58 Calcium nitrate Ca(NO3)2 40+ 2(14+(3x16))=164 What is the relationship between molar mass and formula mass? Molar mass if the formula mass in g Calculating Moles The number of moles present in a weighed sample can be calculated using this equation: Number of moles = mass R.A.M. Similarly mass = moles x R.A.M. 1. How many moles are there in 2. What is the mass of a. 4.4g of carbon dioxide? a. 3 moles of hydrogen gas? b. 80g of methane? b. 0.5 mole of copper(II) oxide? c. 14.2g of chlorine? c. 4 moles of sodium chloride? d. 128g of sulphur? d. 0.3 mole of ethene? e. 1kg of calcium carbonate? e. 0.25 mole helium? Percentage Composition % Z = (Number of atoms of Z) x (atomic Mass of Z) Formula Mass of the compound Calculate the percentage of oxygen in carbon dioxide CO2 12 +(2x16)=44 2 x 16 / 44 = 72.7% parts per million (ppm) This is a way of expressing very dilute concentrations of substances e.g gases in the atmosphere or in exhaust fumes, pollutants in a river, etc. Just as per cent means out of a hundred, so parts per million, or ppm, means out of a million. Convert 0.025% into ppm 0.025 = 100 ? 1000000 1000000 x 0.025 = ? = 250ppm 100 Convert 0.19g/litre into ppm 0.19 = 1000 ? 1000000 1000000 x 0.19 = ? = 190ppm 1000 Past paper questions In 2006, the concentration of carbon dioxide in the atmosphere was 382 ppm. This is equivalent to 382 1000000 = x 100 A halogenoalkane, bromomethane, CH3Br, is a toxic gas used to protect plants against insects. Health and Safety advice states that concentrations above 5 parts per million (ppm) by volume of this gas are harmful. A research laboratory contains 2.5 × 105 dm3 of air. Calculate the maximum volume of bromomethane, in dm3, allowed in the laboratory to comply with the advice given. Jan 09 Exercise 1: Calculation of the Molar Mass of compounds MFE Booklet pp 6-8