Chem 20 – VSEPR and Polarity
advertisement
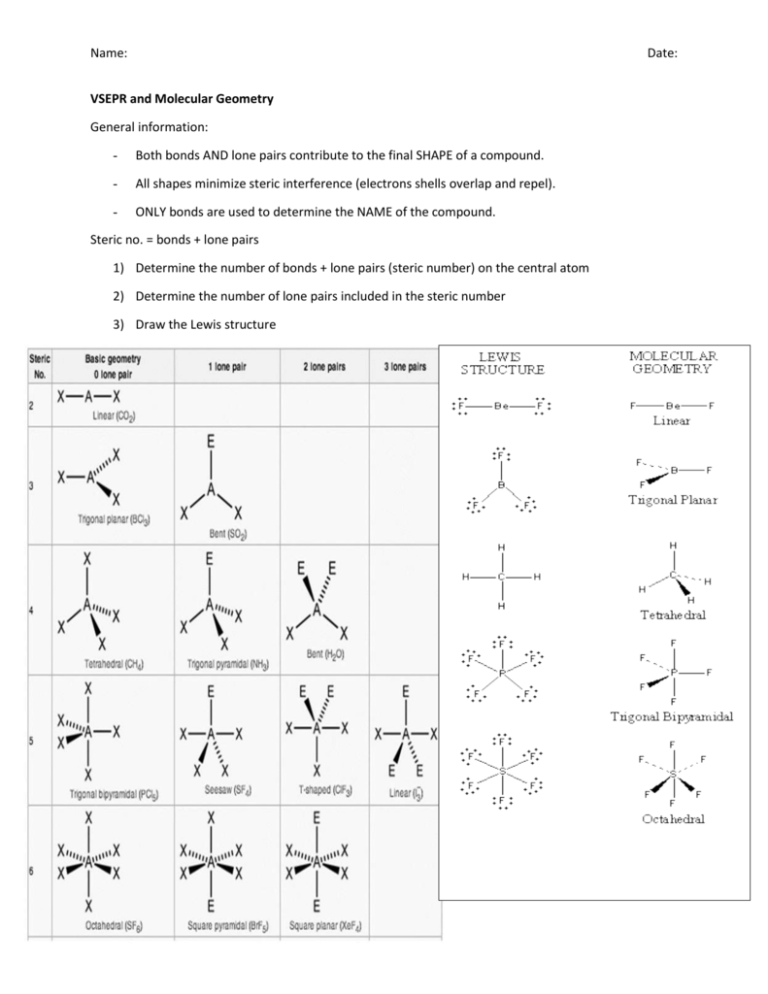
Name: Date: VSEPR and Molecular Geometry General information: - Both bonds AND lone pairs contribute to the final SHAPE of a compound. - All shapes minimize steric interference (electrons shells overlap and repel). - ONLY bonds are used to determine the NAME of the compound. Steric no. = bonds + lone pairs 1) Determine the number of bonds + lone pairs (steric number) on the central atom 2) Determine the number of lone pairs included in the steric number 3) Draw the Lewis structure Name: Date: Name: Date: Step-by-step guide: Lewis dot structures 1: Determine the total amount of valence electrons present in the molecule: CO32- C= 4 O= 6 x 3 = 22 + 2 (remember, that 2- charge means we have an EXTRA 2 electrons) = 24 2: Determine and place the central atom. Surround the central atom with the “outer” atoms (remember, the central atom is the one situated furthest to the left on the periodic table – hydrogen is the exception, as it is never central). O C O O 3: Calculate the number of remaining electrons. Place the remaining electrons around the outer atoms so that each outer atom has 8 electrons (keep in mind that the bond counts as two electrons). Continue doing this until you “run out” of electrons. If you have filled the outer atoms and still have some electrons left over, proceed to place electrons in pairs around the central atom (think about NH3). Electrons remaining = Total electrons – electrons held in bonds Electrons remaining = 24 – 6 Electrons remaining = 18 4: Move lone pairs from the outer atoms to form additional bonds as necessary. Remember, the central atom must always have 8 electrons (unless you are a prick like Boron who only wants 6). How to find the formal charge of each atom: Formal charge = bonds formed – bonds usually formed Formal charge of O =1–2 = -1 How to find bonds usually formed: 1) Draw the Lewis diagram for the atom in question - As you can see, there are two electrons that are not paired up. These are the electrons that are USUALLY available for bonding. Name: Date: Step-by-step guide: Molecular Geometry 1: Draw a rough Lewis diagram for the compound in question: NH3 N= 5 H= 1 x 3 = 8 total electrons H ®® N H H *Remember – bonds contain TWO electrons* 2: Use the rough Lewis diagram to determine the steric number (bonds + lone pairs) - If we examine the diagram above, we can note that there are 3 bonds and 1 lone pair. From this, we can conclude that the steric number for the central atom is 4 (3 bonds + 1 lone pair). PLEASE NOTE THAT DOUBLE BONDS AND TRIPLE BONDS COUNT AS 1 BOND WHEN DETERMINING STERIC NUMBER. 3: Using the molecular geometry chart as a reference, determine the “shape” of the molecule using the steric number: Steric number = 4 - Bonds = 3 - Lone pairs = 1 - Since the compound in question – NH3 – contains a lone pair, we can conclude that the shape of the model is trigonal pyramidal. 4: Draw the final Lewis diagram with proper molecular geometry: 3-D model OR ®® N 2-D model H H H Name: Date: Polarity: In a polar bond, electrons are held more closely by one of the atoms Polarity depends on the electronegativity of the elements and the shape of the molecule Polarity can be determined by finding the difference between the electronegativity of the two atoms. When performing the calculation, always list the atom situated furthest to the right on the periodic table first. IONIC BONDS: Metal and a non-metal Always polar, since the non-metal “holds” all electrons while the metal gives them away Electronegativity difference greater than 1.8 Examples: How to determine the polarity of a compound: Cl-K (3.0 – 0.8 = 2.2) 1: Draw the Lewis structure with the proper molecular geometry. N-Na (3.0 – 0.9 = 2.1) 2: Determine the polarity of each bond by subtracting the electronegativity of the left-most atom from the electronegativity of the right-most atom. POLAR COVALENT: Two or more non-metals Unequal sharing of electrons Electronegativity difference of 0.5 to 1.7 Examples: Cl – C (3.0 – 2.5 = 0.5) O – S (3.5 – 2.5 = 1.0) NON-POLAR COVALENT: Two or more non-metals Equal or almost equal sharing of electrons Electronegativity difference of less than 0.4 Examples: N-N (3.0 – 3.0 = 0.0) Cl-Br (3.0 – 2.8 = 0.2) 3: Examine the shape of the molecule. If the molecule is symmetrical in all directions, it will not be polar. If it is asymmetrical, it is likely polar. To find the total polarity of the compound, separate the molecule into halves and sum the polarities on each side. If there is a difference, we are dealing with a polar compound. Polar Shapes Non-Polar Shapes Bent (one lone pair) Linear Bent (two lone pairs) Trigonal Planar Trigonal Pyramidal Tetrahedral







