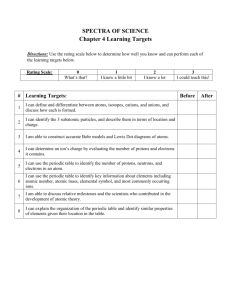
Review for Wednesday`s Quiz
advertisement

Electron Behavior Quiz 1. Explain the three subatomic particles in terms of mass, charge and location. 2. An element has an atomic number of 76. How many protons and electrons are in a neutral atom of the element? 3. All atoms of the same element have the same ____. 4. Isotopes of the same element have different ____. 5. How many protons, electrons, and neutrons does an atom with atomic number 50 and mass number 125 contain? 6. In Bohr's model of the atom, where are the electrons and protons located? 7. How many f orbitals are there in any single energy level in an atom? d orbitals? p orbitals? 8. What is the maximum number of electrons in the second principal energy level? 9. What is the next atomic orbital in the series 1s, 2s, 2p, 3s, 3p? 10. What is the electron configuration of potassium? 11. How many electrons can the first energy level hold? The second energy level? The third energy level? The fourth energy level? 12. Name the “Three Techniques to Represent an Atom”. 13. Use the element Selenium, 34 to show each of the techniques in #12. 14.