Steps for Drawing Dot Structures for Covalent Bonds
advertisement

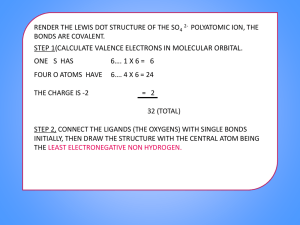
Steps for dot structures for covalent bonds 1. Find how many bonds you need to make a full out shell (in the case of H and He), or an octet. H needs 1 bond and no other electrons ex H Group 4 needs 4 bonds ex C Group 5 needs 3 bonds Ex N Group 6 needs 2 bonds Ex O Group 7 needs 1 bond Ex F 2. Determine you central atom (C is always a central atom; hydrogen is never a central atom). The central atom is typically the first atom in the formula and very often there is only one of them. 3. Arrange the other atoms around the central atom. In CF2Cl2, Carbon is the central atom, the other atoms go around it F F C Cl Cl 4. How many bonds does each need? Each F needs 1, each Cl needs 1, and each C needs 4 so… F F C Cl Cl 5. Then draw the rest of the electrons to make the octet F F C Cl Cl 6. When you are done you can check your work by A. Summing the total amount of valance electrons in the formula C has 4 valence electrons 4X1=4 F has 7 valence electron 7 X 2 = 14 Cl has 7 valence electrons 7 X 2 = 14 Total 32 valence electrons B. Count how many valence electrons are in your drawing remembering that the “bond” is worth two. You should have the same amount. If you have more, then you need to add a double or triple bond somewhere.