Basic Atomic Structure
advertisement

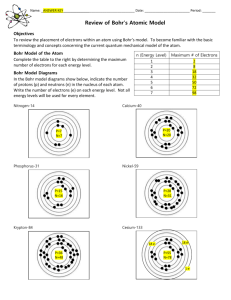
Basic Atomic Structure What does the atom look like? Democritus theorized a solid, indestructible object His atom looked something like this: It wasn’t until J.J.Thompson came around, that the theory of atomic structure changed. Why? Cool, huh? This was the primary idea of the structure of an atom for almost 2000 years. The discovery of protons & electrons!!! The Thompson Model: called the “Plum-pudding” Model Scattered electrons “Ball” of positive charge, with electrons randomly scattered throughout. Cloud of Positive charge Rutherford Model: With the discovery of the nucleus, Protons are localized in the center of the atom, and electrons are circled around them. Bohr Model: Bohr theorized that electrons followed specific pathways around the nucleus: called “orbitals” While this model is probably most familiar… it STILL isn’t accurate! The Quantum Mechanical Model: This model states that electrons still follow specific pathways, these orbitals, have specific 3D shapes: It also says that because you can never know the speed of an electron & its position at the same time, you could only guess where an electron was likely to be 90% of the time: What you need to know about: subatomic particles Protons- positive charge, in the nucleus, has a mass of 1 amu Neutron- neutral/no charge, in the nucleus, has a mass of 1 amu Electron- negative charge, towards the outside of the atom, has no mass










![The electronic configuration of phosphorus is [Ne] 3s2 3p3](http://s3.studylib.net/store/data/008974852_1-8381577ce936fbfa611892c1a5f109cd-300x300.png)