Lewis Structures & Metallic Bonding Chemistry Presentation
advertisement
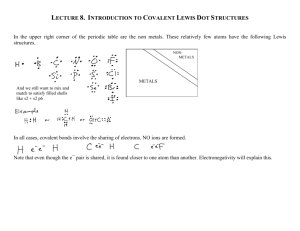
Tuesday 10/19/10 Return Naming Compounds Quiz Collect Symbol Me Projects Lewis structures Metallic Bonding Hydrogen Bonding Jeopardy Review Atoms form bonds to gain a full shell of valence electrons. (Octet Rule) If electrons are not involved in bonding, then they are lone pairs. Each bond is made up of 2 electrons. Count up the total # of valence electrons. If it’s an ion, then add or subtract e- as needed. Connect all the atoms with single bonds. Place the remaining electrons as lone pairs around the atom. Create double or triple bonds if there are not enough electrons to go around. ALWAYS FOLLOW THE OCTET RULE!!!! Draw the Lewis structure for NO3- Draw the Lewis structure for CCl3+ Draw the Lewis structure for CO2 Draw the Lewis structure for N2O4 Draw the Lewis structure for C2N24- Draw the Lewis structures for PO43- Draw the Lewis structure for OCN- http://www.youtube.com/watch?v=lkl5cbfqF RM&feature=related\ http://www.youtube.com/watch?v=LGwyBeu VjhU