word - Seattle Central College
advertisement

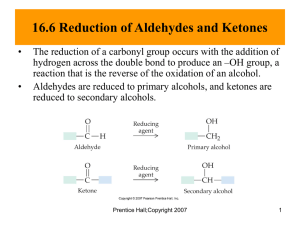
CHEM 122: Introduction to Organic Chemistry Chapter 9: Aldehydes and Ketones. 1. Following are structural formulas for two steroid hormones. Cortisone Aldosterone a) Name the functional groups in each. b) Mark all stereocenters in each hormone and state how many stereoisomers are possible for each. 2. Draw structural formulas for the four aldehydes with the molecular formula C5H10O. Which of these aldehydes are chiral? 3. Draw structural formulas for these aldehydes. a) b) c) d) e) f) Formaldehyde Propanal 3,7-Dimethyloctanal Decanal 4-hydroxybenzaldehyde 2,3-Dihydroxypropanal 4. Draw structural formulas for these ketones. a) b) c) d) e) f) Ethyl isopropyl ketone 2-Chlorocyclohexanone 2,4-Dimethyl-3-pentanone Diisopropyl ketone Acetone 2,5-Dimethylcyclohexanone 5. Write the IUPAC names for these compounds. CHO CHO O CHOH O a) OH b) CHOH c) CH 2 OH d) NH 2 6. Explain why each name is incorrect. Write the correct IUPAC name for the intended compound. a) b) c) d) 2-Pentanal Cyclopentanal 3-Ethyl-2-butanone 5-Aminobenzaldehyde 7. Acetone is completely soluble in water, but 4-heptanone is completely insoluble in water. Explain. 8. Account for the fact that acetone has a higher boiling point (56oC) than ethyl methyl ether (11oC) even though their molecular weights are almost the same. 9. Propanal (bp 49oC) and 1-propanol (bp 97o) have about the same molecular weight, yet their boiling points differ by almost 50oC. Explain this fact. 10. Draw a structural formula for the principal organic product formed when each compound is treated with Tollens’ reagent. If there is no reaction, say so. a) b) c) d) Butanal Benzaldehyde Cyclohexanone Cyclohexanol 11. Suppose that you take a bottle of benzaldehyde (a liquid, bp 179oC) from a shelf and find a white solid in the bottom of the bottle. The solid turns litmus red; that is, it is acidic. Yet aldehydes are neutral compounds. How can you explain these observations? 12. Write a structural formula for the principal organic product formed by treating each compound with NaBH4 followed by H2O. O O a) CH3CCH2CH3 b) CH3(CH2)4CH O O CH CH 3 c) OH d) 13. Draw a structural formula for the product formed by treatment of butanal with each set of reagents. a) b) c) d) H2/metal catalyst NaBH4, then H2O Ag(NH3)2+ (Tollens’ reagent) K2Cr2O7/H2SO4 14. Draw a structural formula for the product formed by treatment of acetophenone, C6H5COCH3, with each set of reagents. a) b) c) d) H2/metal catalyst NaBH4, then H2O Ag(NH3)2+ (Tollens’ reagent) K2Cr2O7/H2SO4 15. Draw all enol forms of each aldehyde and ketone. O a) CH3CH2CH O CH 3 c) O b) CH3CCH2CH3 16. Which compounds are hemiacetals, which acetals, and which are neither? OCH 3 CHOCH 3 a) b) O d) CH3CH2CHOCH3 O CH2CH3 O c) OCH 2 CH 3 CH3OCH2OCH3 OH O e) f) 17. Draw the hemiacetal and then acetal formed in each reaction. In each case, assume an excess of the alcohol. a) Propanal + methanol b) Cyclopentanone + methanol 18. Draw the structures of the aldehydes or ketones and alcohols formed when these acetals are treated with aqueous acid and hydrolyzed. OCH 3 OCH 3 O a) b) OCH 3 O OCH 3 O OCH 3 c) OCH 3 d) 19. List the reagents and experimental conditions to convert cyclohexanone to each of the following compounds. 20. Draw a structural formula for an aldehyde or a ketone that can be reduced to produce each alcohol. If none exists, say so. CH 3 CH2 OH H3C OH a) b) c) COH CH 3 d) HO OH 21. Show how to bring about these conversions. In addition to the given starting material, use any other organic or inorganic reagents as necessary. 22. Describe a simple chemical test by which you could distinguish between the members of each pair of compounds. a) Cyclohexanone and aniline b) Cyclohexene and cyclohexanol c) Benzaldehyde and cinnamaldehyde 23. 5-Hydroxyhexanal forms a six-membered cyclic hemiacetal, which predominates at equilibrium in aqueous solution. a) Draw a structural formula for this cyclic hemiacetal. b) How many stereoisomers are possible for 5-hydroxyhexanal? c) How many stereoisomers are possible for this cyclic hemiacetal? 24. The following molecule is an enediol; each carbon of the double bond carries an –OH group. Draw structural formulas for the -hydroxyketone and the -hydroxyaldehyde with which this enediol is in equilibrium. -hydroxyaldehyde HC OH C OH CH 3 An enediol -hydroxyketone 25. Alcohols can be prepared by the acid-catalyzed hydration of alkenes and by the reduction of aldehydes and ketones. Show how you might prepare each of the following alcohols by 1) acid-catalyzed hydration of an alkene and 2) reduction of an aldehyde or a ketone. a) b) c) d) Ethanol Cyclohexanol 2-Propanol 1-Phenylethanol 26. Glucose, C6H12O6, contains an aldehyde group but exists predominantly in the form of the cyclic hemiacetal shown here. We will discuss this cyclic form of glucose in Chapter 12. CH 2OH O OH H H OH H HO H H OH -D-Glucose A cyclic hemiacetal is formed when the –OH group of one carbon bonds to the carbonyl group of another carbon. Which carbon in glucose provides the –OH group and which provides the CHO group?