ALDEHYDES AND KETONES
advertisement

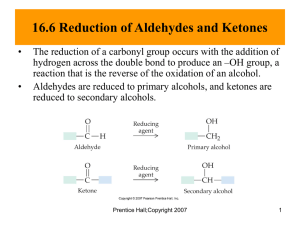
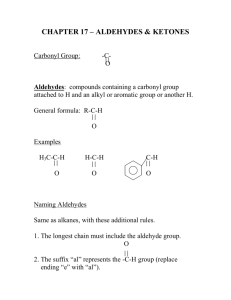
ALDEHYDES AND KETONES Aldehydes and Ketones H R R C O Aldehyde C O R Ketone bond - two overlapping 2p orbitals H H C O bond bond- overlapping 1s H-orbital and sp2 C-orbital lone Pairs H d+ d 118o C O H3C 121o H 118o H C C H 121o H Resonance Structures H H Most Reactive Group – C O C O electrons + polarisation H3C H3C Names al – aldehydes, one - ketones H H C O C O H Methanal (formaldehyde) H H3C H C O CH3CH2 Propanal Ethanal (acetaldehyde) C O CH3CH2CH2CH2 Pentanal Nomenclature of Aldehydes If the aldehyde group is attached to a ring, Nomenclature of Ketones Oxidation An aldehyde has a greater partial positive charge on its carbonyl carbon than does a ketone • Steric factors contribute to the reactivity of an aldehyde • The carbonyl carbon of an aldehyde is more accessible to the nucleophile • Ketones have greater steric crowding in their transition states, so they have less stable transition states Reactions 1. Redox reactions RCH O H RC O H2 red. KMnO4 or CrO3 O R C OH RCH2OH R C O R' H2 R H C R' OH 2. Addition: AN Addition of water H OH H C O + H2O R Aldehyde hydrate C R (ketone: OH Addition of bisulfite R C R' SO3-Na+ R O + NaHSO3 Bisulfite C R' OH ) Addition of NH3 H NH2 H C O + NH3 R C Aldehyde ammonia (ketone: R OH R C N Addition of HCN R C R' Cyanohydrine C O + HCN R' OH ) 3. Condensation reactions (addition followed by elimination) Ammonia derivatives R R H C N O+ Y N Y C N R" R' H R' C Primary amine R C R' R H N O+ H R" R' Schiff base Hydroxylamine R R H C N O+ C OH OH Oxime R' H R' N Hydrazine R C R' H H N O+ H R C N H N NH2 R' Hydrazone Phenylhydrazine R H C O+ H R' H N N Phenylhydrazone 2,4-dinitrophenylhydrazine H R H C O+ H R' N N NO2 NO2 2,4-dinitrophenylhydrazone Aldol condensation CH2 C H + CH2 C H R O R' O Aldol OH- CH2 CH CH C H R OH R' O Reaction with alcohol O R C H + HO R' OH R C O R' Hemiacetal (addition!) O R' Acetal (condensation!) H O R R C H H + HO R' R C H O R' Keto–Enol Tautomerism Oxidation of a Primary Alcohol Reduction by Addition of a Hydride Ion and a Proton Aldehydes and ketones react with nucleophiles to form addition products: nucleophile addition reactions Water adds to an aldehyde or ketone to form a hydrate Hydration of Aldehydes and Ketones R C O •• •• R' HOH R •• HO •• C R' •• O •• H Alcohols Under Analogous Reaction with Aldehydes and Ketones R C O •• •• R' R"OH R •• R"O •• C R' •• O •• H Product is called a hemiacetal. Example O CH + 2CH3CH2OH HCl CH(OCH2CH3)2 + H2O Benzaldehyde diethyl acetal (66%) Reaction with Primary Amines: Imines If the nucleophile that adds to the aldehyde or ketone is an O or an N, a nucleophilic addition–elimination reaction will occur Imine (Schiff's Base) Formation H2N •• + C O •• •• •• HN C •• O •• R R a carbinolamine •• N R C (imine) + H2O H Example O CH + CH3NH2 CH=NCH3 + H2O N-Benzylidenemethylamine (70%) Reaction with Derivatives of Ammonia H2N G + R2C H2N OH hydroxylamine O R2C NG + H2O R2C NOH oxime Reaction with Derivatives of Ammonia H2N G + R2C H2N OH O R2C NG + H2O R2C NOH hydroxylamine oxime H2N R2C NH2 hydrazine etc. NNH2 hydrazone Example O C + H2NNH2 NNH2 C (73%) + H2 O Example O CCH3 + H2NNH phenylhydrazine NNH CCH3 a phenylhydrazone (87-91%) + H2 O An aldol addition product loses water to form an aldol condensation product Aldol Condensation O O NaOH 2RCH2CH RCH2CH OH CHCH R Aldol Addition of Acetaldehyde O 2CH3CH O NaOH, H2O 5°C CH3CH CH2CH OH Acetaldol (50%) Hemiacetal reacts further in acid to yield an acetal R •• R"O •• C •• OR •• Product is called an acetal. R' ROH, H+ R •• R"O •• C R' •• O •• H Product is called a hemiacetal. Formaldehyde O HCH formaldehyde cannot form an enolate formaldehyde is extremely reactive toward nucleophilic addition Acrolein O H2C CHCH Acrolein O H2C CHCH Acrolein O H2C CHCH