File
advertisement

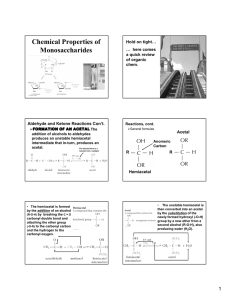
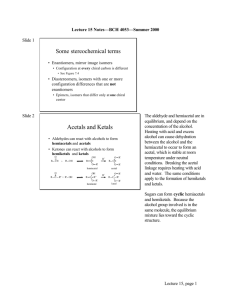
1. You should know the following terms: constitutional isomer, stereoisomers, enantiomers, diastereomers, epimers, anomers, and mutarotation. Make sure you understand how to apply these terms to carbohydrate structures.: a. Isomer: compounds that have the same molecular formula but different structures (BOTH stereoisomers and constitutional isomers) b. Constitutional isomers: differ in the order of attachment of atoms c. Stereoisomers: atoms are connected in the same order but differ in spatial arrangement d. Enantiomers: stereoisomers that are nonsuperimposable mirror images e. Diasteriomers: stereoisomers that are not mirror images f. Epimers: diastereomers that differ at one of several asymmetric Carbon atoms g. Anomers: diastereomers that differ at a new asymmetric Carbon atom formed on a ring closure h. Mutarotation: the interconversion of - and - anomers 2. You need to know the following organic functional groups: aldehyde, ketone, ester, hemiacetal, acetal, hemiketal, ketal, and phosphoester: a. Aldehyde: b. Ketone: c. Ester: d. Hemiacetal: e. Acetal: f. Hemiketal: g. Ketal: h. Phosphoester: (phosphodiester) i. Acetal/Ketal formation (3rd product is hemiketal/hemiacetal 7th product is acetal/ketal) O R H R' O C H 3O H HO H OCH R R' HO OCH R R C H 3O R 3 - H HO R H 3 H 2O R R' OCH 3 R C H 3O H 3 R' H OCH R' C H 3O R 3 - H H R' H OCH C H 3O R 3 R' OCH 3 R' OCH 3 R' OCH H R' OCH HO R R' H 2O R H R R O 3 R' H OCH 3 R' OCH R' 3 3. You should understand the various general nomenclature for carbohydrates e.g. aldose, ketose, pyranose, furanose, glucopyranose, and fructofuranose. a. Classification: i. Monosaccharide (simple sugar) ii. Complex Carbohydrates: 2 + sugars 1. Disaccharides: 2 monosaccharides 2. Oligosaccharides: 3-10 monosaccharides 3. Polysaccharides: 10 + monosaccharides b. Name: based on number of Carbon atoms i. Ex. 5 = pentose 6 = hexose c. Categories i. Aldose [ Cn(H2O)n ]: a sugar that contains 1 aldehyde per molecule 1. Ex. ii. Ketose: a sugar that contains 1 ketone per molecule 1. Ex. iii. Pyranose: a sugar that includes a six-member ring made up of 5 Carbons and 1 Oxygen 1. 2. Ex. iv. Furanose: a sugar that includes a five-member ring made up of 4 Carbons and 1 Oxygen 1. 2. Ex. v. Glucopyranose: glucose in a pyranose form 1. See Example III (pyranose) vi. Fructofuranose: fructose in a furanose form 1. Ex. 4. You only need to know the Fischer projections for D--glucose, D-mannose, and D-- galactose. (You should also know the Haworth and chair structures for both alpha and beta D--glucose.) a. Fischer Projections: i. D-glucose: ii. D-mannose: iii. D-galactose: b. Hawthorne Projections: i. -D-glucose: ii. -D-glucose: c. Chair: i. -D-glucose: ii. -D-glucose: 5. You should know how to convert a Fischer projection into a Haworth projection or convert a Haworth projection into a Fischer projection. You should also be able to convert a Haworth projection into a chair conformation. See the Fischer Projection review. Remember, the most stable chair conformation has the OH and CH2OH groups for glucose equatorial (except for the anomeric OH group which may either be axial or equatorial). There will definitely be some questions like this on my part of the future exams: a. For chair conformations look at 4 6. You should be able to assign R/S designations for carbohydrate stereocenters. (the atom with the higher atomic number has priority) 7. Know the designation for the anomeric groups as either being alpha or beta. a. Alpha: anomeric R group is in the axial position b. Beta: anomeric R group is in the equatorial position 8. Understand the anomeric effect and its M.O. basis: a. Anomeric effect: overlap between the ring Oxygen’s lone pair bonding orbital and the Carbon/Oxygen antibonding orbital b. MO theory: theory that descirbes the area an electron will occupy in a molecule based of the molecule’s wave function 9. Know the mechanism for the formation of a hemiacetal and acetal. Look at section 2 i 10. Know the reactions we covered in class: oxidation/reduction, isomerization, esterification, amino acid derivatives, and glycoside formation: a. oxidation/reduction: OIL/RIG b. isomerization: changes stereochemistry i. ex. c. esterification: The OH groups of sugars can react with phosphoric acid to give phosphate esters d. AA derivatives: attaching amino acid residue to glycan e. Glycoside formation: form bond between anomeric carbon of certain glycans and Nitrogen/Oxygen of other types of molecules (N-/Oglycosidic bond) 11. Know the structures for D--gluconic acid, D--glucoronic acid, D--glucaric acid, and the general alditol structures: a. General: sugar that is a polyalcohol formed by the reduction of the carbonyl group of monosaccharide b. D-gluconic acid c. D-glucoronic acid d. D-glucaric acid e. 12. You should be able to recognize whether cyclic carbohydrates have beta 1,4 or alpha 1,4 linkages as well as other glycoside bond variations. Some examples discussed in class that illustrate these bonding patterns were sucrose, lactose, maltose, starch, cellobiose, and cellulose: (based on anomeric Carbon) a. General i. Alpha linkage: ii. Beta linkage: b. Sucrose: (glucose + fructose) c. Lactose: (glucose + galactose) d. Maltose: e. Starch: f. Cellobiose: g. Cellulose: 13. Understand the concept of functionalizing with UDP: 14. Know the meaning of a reducing sugar and be able to identify them a. Reducing sugar: sugars that react with oxidizing agents (Cu2+) 15. Other terms you should know include proteoglycan, amino sugar, and glycoprotein Sugar + macromolecule: a. Proteoglycan: a heavily glycosylated protein b. Amino sugar: a sugar containing an amine group in placed of a hydroxyl group c. Glycoprotein: proteins that contain oligosaccharide chains covalently attached to polypeptide side chains 16. You should read about erythropoietin but do not need to study the blood groups and lectin 17. You should know the meaning of amylase, pectin, glycogen, and homopolymer a. Amylase: enzyme that catalyzed breakage of glycosidic bonds into simple sugars (in human saliva) b. Pectin: heteropolysaccharide contained in cell wall of plants c. Glycogen: storage form of glucose made by glycosidic bonds d. Homopolymer: product created when a single monomer is polymerized into a macromolecule