Name: Chemistry A Date: Period: Identify the Trend
advertisement

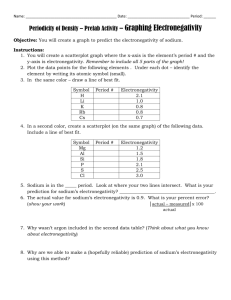
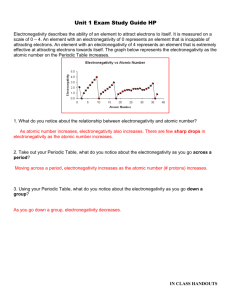
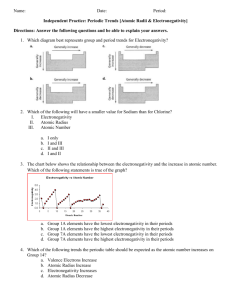
Name: Chemistry A Date: Period: Identify the Trend – Electronegativity and the Periodic Table Electronegativity describes the ability of an element to attract electrons to itself. It is measured on a scale of 0 – 4. An element with an electronegativity of 0 represents an element that is incapable of attracting electrons. An element with an electronegativity of 4 represents an element that is extremely effective at attracting electrons towards itself. The graph below represents the electronegativity as the atomic number on the Periodic Table increases. 1. What do you notice about the relationship between electronegativity and atomic number? 2. Take out your Periodic Table, what do you notice about the electronegativity as you go across a period? 3. Using your Periodic Table, what do you notice about the electronegativity as you go down a group? IN CLASS HANDOUTS Name: Chemistry A Date: Period: Above is the Periodic Table with the electronegativity values for every element. Examine the image above and identify any trends that you see emerging. Remember, the closer to 0, the worse the element is at attracting electrons. 4. You can see that as you go down a group the electronegativity of the element decreases. Using what you know about Bohr electron structures, why do you think electronegativity decreases down a group? If you are confused, draw some Bohr diagrams to help work out your answer! 5. You can see that as you go across a period the electronegativity of the element increases. Using what you know about Bohr electron structures, why do you think electronegativity increases across a period? If you are confused, draw some Bohr diagrams to help work out your answer! Also, think about how the nucleus changes across a group! IN CLASS HANDOUTS Name: Chemistry A Date: Period: Final Review Question: Look at the test question. What do you think it is now? 30. (Level 4. Advanced Difficulty) A friend and you are running a lab where you are studying one of the Periodic Trends that was covered during Unit 1. Once you are finished your friend makes the following graph. When you go home that night you realize that your incompetent friend forgot to label the y-axis. Using your knowledge of Periodic Trends, explain which trend (ionization energy, electronegativity, and atomic radius) is illustrated by the graph above. You must justify your answer with evidence to gain full credit. IN CLASS HANDOUTS