Electronegativity Tug of War
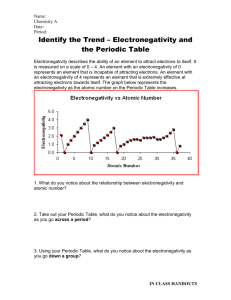
advertisement

Name_______________________________________________________Period_____________ TUG OF WAR TIME It’s time to settle who’s the strongest. Your teacher will select 7 teams of students to stand off in a Tug of War. Teams A-G Will Do Battle Today. May the Strongest “Teams” Win. 1. 1. Draw the 1st tug of war and be 2. 2. How did the 2 teams match 3. 3. Why did they match up sure to show which team won(Team up? this way? A or B) : 4. Draw the 2nd tug of war and be sure to show which team won (C and D): 5. How did the 2 teams match up? 6. Draw the 3rd tug of war and be sure to show which team won (E and F): 7. How did the 2 teams match up? 6. Why did they match up this way? 8. Why did they match up this way? 9. a. Rank the teams in order of strength on a scale from 0.0(super-wimps) to 4.0(Strongest Possible): b. Explain why each team received this ranking TEAM A = TEAM TEAM C TEAM D TEAM E = TEAM F = TEAM G = B= = = WHY? WHY? WHY? WHY? WHY? WHY? WHY? 10. Even though Team G never got a chance to battle another team in a Tug of War, how were you able to come up with a rank for Team G. Electronegativity: (Electron Hunger) Scientists have observed that atoms also “fight” or compete for electrons. An atom’s “goal” being to have a complete valence shell of electrons to shield its nucleus from outside forces. Scientists have also come up with scale that ranks an atom’s ability to attract or pull electrons towards the atom’s nucleus. The ability of an atom to attract electrons is called its Electronegativity (or Electron Affinity). We can also look at Electronegativity as e- Hunger. Scientists use a scale ranging from 0.0 to 4.0 to represent the strength of various atoms. This is determined by the pull the atom has on electrons when bonding with other elements in a Tug-of-War for those electrons involved in the bond. The hungrier an element is, the stronger it can pull on those electrons, so the closer it will hold those electrons to its nucleus. 11. Take a look at your periodic table: Draw the Bohr Li F He models for: What would it take to make this atom Stable (Happy) List the atoms Electronegativity This atoms electronegativity is: (High or Low) Why is this atoms electronegativity High or Low? 12. How does electronegativity relate to Valence Shell e-s? 13. How does electronegativity relate to the # of protons in an atom’s nucleus? 14. What does it mean if an atom’s Electronegativity equals: 0.0 = 0.8 = 4.0 = 15. Why are the electronegativity’s of the Noble Gases in Group 8 so low? 16. Why do Group 1 elements have such low electronegativity’s? 17. Why do Group 7 elements have such a high electronegativity’s? Back to our tug of war: Electronegativity Difference = A TUG of WAR for e-s Calculating the Electronegativity Difference (Tug-of-War) allows you to determine how electrons are being shared: equally, unequally, or if they are being stolen. All you have to do is find the difference between the two elements involved in the bond. (aka. Subtract their Electronegativity numbers.) The Electronegativity Difference can then tell you what kind of bond will form. 18a. How is our Tug-of-War activity similar to two atoms bonding? b. What do the 2 teams represent? c. What do the 2 flags represent? d. What do the pullers represent and what happens if you have more pullers? e. Why don’t all of the teams get to use the same number of pullers? 19. Which Team (A-G) was the: strongest = 19a. If the strongest b. Where on the periodic team was an atom, table would you find really which elements on hungry or strongly attractive the periodic would elements? (Those with High that team best Electronegativity’s) represent? 15 20a. If the weakest team was an atom, which elements on the periodic would that team best represent? b. Where on the periodic table would you find really weak or less attractive elements? (Those with Low Electronegativity’s): weakest = c. What type of elements d. Why are these have really High atoms able to attract Electronegativity’s? (Who e- so strongly? are the e- thieves?) c. What type of elements d. Why are these have really Low atoms not able to Electronegativity’s? (Who attract e- strongly? are the e- losers?) 21. What elements on the periodic table are best represented by Teams A-G and Why? TEAM A = TEAM B = TEAM C = TEAM D = TEAM E = TEAM F = WHY? WHY? WHY? WHY? WHY? WHY? 22a. Why didn’t Team G get a chance to play in the Tug of War? b. What is Team G’s real Electronegativity? c. Which elements would Team G represent pretty well? The Electronegativity Difference can then tell you what kind of bond will form: Calculating the Electronegativity Difference (Tug-of-War) allows you to determine how electrons are being shared: equally, unequally, or if they are being stolen. All you have to do is find the difference between the two elements involved in the bond. (aka. Subtract their Electronegativity numbers.) There are 3 major types of bonds that are created due to these differences: Non-Polar Covalent, Polar Covalent, and Ionic Bonds Calculate the Electronegativity Difference for each of the bonds above: Type of Bond Electronegativity Difference Non-Polar Covalent Polar Covalent Ionic < 0.5 0.5-2.0 >2.0 Shared Equally Shared Unequally (One atom hungrier and greedier) Electron Transferred (e- Stolen or Lost) Diagram Electrons Simplified Diagram + 23. When two atoms of the same element bond, they form a ______________________ molecule. 24. The molecule is covalent because ________________________________________________. 25. Sketch and Explain the bonding between two Cl atoms (Cl-Cl) Drawing Bond Type Explanation 26. What type of bond is formed between atoms that share electrons equally? ___________________ 27. ___________________ is the most electronegative element and is assigned a value of 4.0. This makes it the hungriest element. 28. Where does fluorine (F) reside on the periodic table ___________________________ 29. True or False: There are other elements that can attract an electron better than Fluorine._________ 30. Sketch and Explain the bonding between H-F and describe who wins. Drawing Bond Type Explanation 31. What type of bond is formed from the unequal sharing of electrons? _________________________ 32. A molecule that has separation of charge, a region of + and a region of -, is called _____________. 33. The most famous example of this type of molecule is_________________________ 34. Sketch and Explain the bonding between Li-Cl. Drawing Bond Type Explanation 35. Briefly describe how ions are formed: 36. Why is the compound formed by an ionic bond not referred to as a molecule? 37. Complete the table below. Make a diagram to show how each pair of atoms listed shares or transfer electrons and determine the type of bond by showing your calculations of electronegativity differences. (NOTE: Always subtract the smaller electronegativity value from the larger one. You should never have a negative value! Pairs of Atoms Diagram Calculation of Bond Type Electronegativity H-O bond C-O bond K-F bond C-H bond Br-Br bond 38. Why do some atoms have higher electronegativity’s than other atoms? (Provide examples) 39. Why do some atoms have electronegativity’s that equal 0? (Provide examples) 40. Why do atoms have much weaker electronegativity’s? (Provide examples) 41. What atomic characteristics determine an atoms electronegativity: Come up with at least 3: 1. 2. 3. 41. Study the electronegative values on your periodic table and try to find the trends. a. a. Where are electronegativity values the lowest? b. b. Where are electronegativity values the highest? c. c. What is the trend in the electronegativity values as you go across a period? d. d. What is the trend in the electronegativity values as you go down a family? Using Arrows: Draw and Label the overall pattern (trend) for electronegativity on the periodic table below 42. How can knowing this pattern help you to predict what will happen when you mix different elements? 43. Why did we play tug of war at the start of class? How could it help you to remember how atoms work? MVP ( Explain the Most Valuable point of this entire activity and assignment)