Unit 1 Exam Study Guide HP Electronegativity describes the ability
advertisement
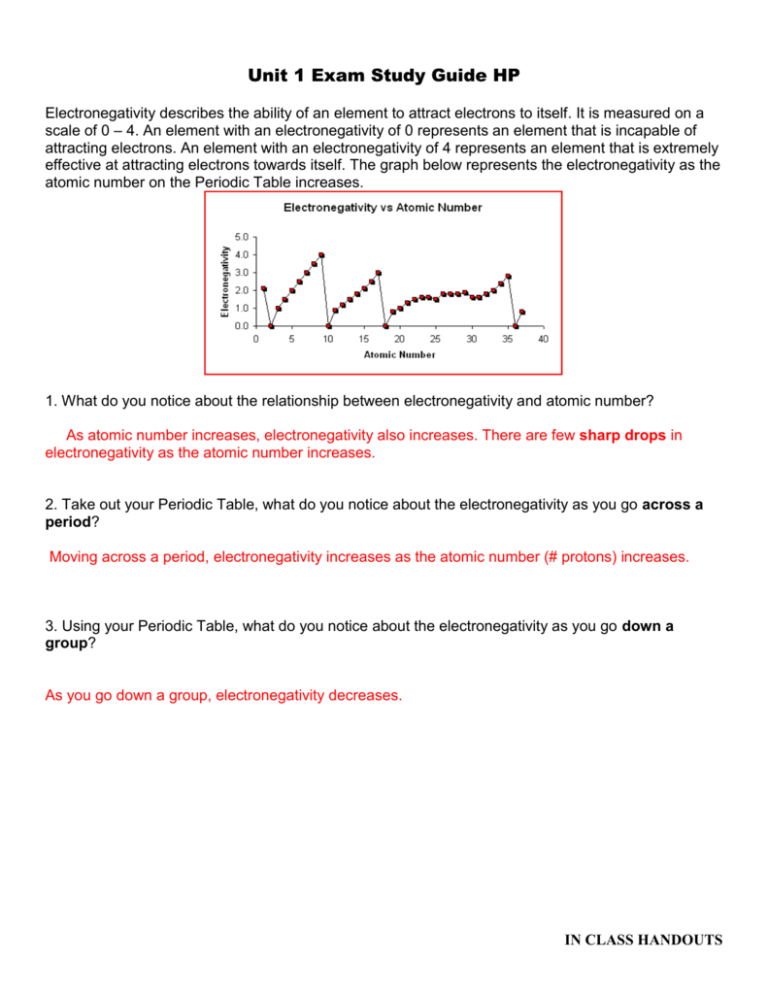
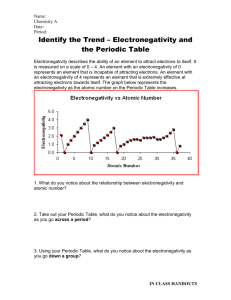
Unit 1 Exam Study Guide HP Electronegativity describes the ability of an element to attract electrons to itself. It is measured on a scale of 0 – 4. An element with an electronegativity of 0 represents an element that is incapable of attracting electrons. An element with an electronegativity of 4 represents an element that is extremely effective at attracting electrons towards itself. The graph below represents the electronegativity as the atomic number on the Periodic Table increases. 1. What do you notice about the relationship between electronegativity and atomic number? As atomic number increases, electronegativity also increases. There are few sharp drops in electronegativity as the atomic number increases. 2. Take out your Periodic Table, what do you notice about the electronegativity as you go across a period? Moving across a period, electronegativity increases as the atomic number (# protons) increases. 3. Using your Periodic Table, what do you notice about the electronegativity as you go down a group? As you go down a group, electronegativity decreases. IN CLASS HANDOUTS Above is the Periodic Table with the electronegativity values for every element. Examine the image above and identify any trends that you see emerging. Remember, the closer to 0, the worse the element is at attracting electrons. 4. You can see that as you go down a group the electronegativity of the element decreases. Using what you know about Bohr electron structures, why do you think electronegativity decreases down a group? If you are confused, draw some Bohr diagrams to help work out your answer! As you move down a group, you are adding more and more orbitals to the electron cloud (as each element is in a different period). Nucleus of an atom is shielded by these orbitals, so the attraction between the nucleus and electrons (from another atom) decreases. Electrons are too far away from the nucleus due to the added orbitals. 5. You can see that as you go across a period the electronegativity of the element increases. Using what you know about Bohr electron structures, why do you think electronegativity increases across a period? If you are confused, draw some Bohr diagrams to help work out your answer! Also, think about how the nucleus changes across a group! As you move across the period, added proton in the nucleus increases the nuclear charge of an atom. Due to an increase in the nuclear charge, the atom is able to attract electrons (from another atom) a lot more efficiently. 6. Write the electron configuration and the abbreviated configuration for the following elements: a) Cu : 1s22s22p63s23p64s23d9 [Ar] 4s23d9 b) Ca: 1s22s22p63s23p64s2 [Ar] 4s2 7. Which element is represented by the following configurations: a) 1s22s22p63s23p64s23d8 Fe #28 8. Write the orbital notation for Calcium. IN CLASS HANDOUTS ____ ____ 1s 2s ____ ____ _____ 2p _____ 3s _____ _____ _____ 3p _____ 4s 9. Identify the element based on the orbital notation: Phosphorus #15 Essay Topic: Electron configuration, orbital notation Periodic Trends based on nuclear charge/orbitals Contribution of Thompson, Rutherford, and Bohr to the development of modern atomic theory. IN CLASS HANDOUTS