HW: Periodicity of Density Prelab - Liberty Union High School District
advertisement

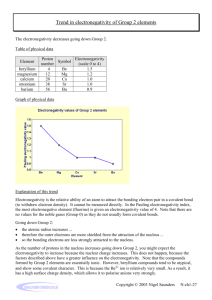
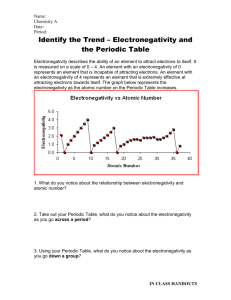
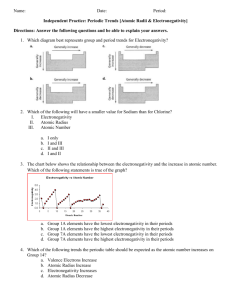
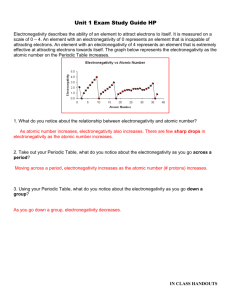
Name: ____________________________________________ Date: ______________________________ Period: ______ Periodicity of Density – Prelab Activity – Graphing Electronegativity Objective: You will create a graph to predict the electronegativity of sodium. Instructions: 1. You will create a scatterplot graph where the x-axis is the element’s period # and the y-axis is electronegativity. Remember to include all 5 parts of the graph! 2. Plot the data points for the following elements . Under each dot – identify the element by writing its atomic symbol (small). 3. In the same color – draw a line of best fit. Symbol H Li K Rb Cs Period # Electronegativity 2.1 1.0 0.8 0.8 0.7 4. In a second color, create a scatterplot (on the same graph) of the following data. Include a line of best fit. Symbol Mg Al Si P S Cl Period # Electronegativity 1.2 1.5 1.8 2.1 2.5 3.0 5. Sodium is in the _____ period. Look at where your two lines intersect. What is your prediction for sodium’s electronegativity? __________________________________________. 6. The actual value for sodium’s electronegativity is 0.9. What is your percent error? (show your work) │actual – measured│x 100 actual 7. Why wasn’t argon included in the second data table? (Think about what you know about electronegativity) 8. Why are we able to make a (hopefully reliable) prediction of sodium’s electronegativity using this method?