Check for Understanding: Explain charge and where it comes from
advertisement

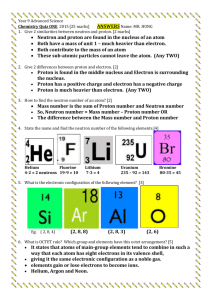
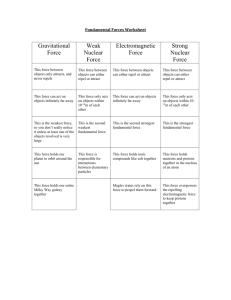
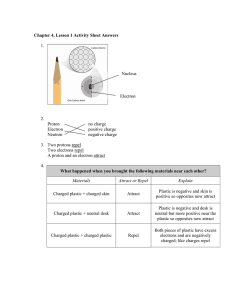
Check for Understanding: Explain charge and where it comes from. 1. Which part of the atom is positive? Which part of the atom is negative? Which part of the atom is neutral? 2. For each statement determine if the particles will repel or attract. Two positive charges will… Two negative charges will… A positive and a negative charge will… An electron and a proton will… An electron and a neutron will… 3. How can you make something negatively charged? 4. How can you make something positively charged? Check for Understanding: Explain charge and where it comes from. 1. Which part of the atom is positive? Which part of the atom is negative? Which part of the atom is neutral? Proton – positive Neutron – neutral Electron - negative 2. For each statement determine if the particles will repel or attract. Two positive charges will… repel Two negative charges will… repel A positive and a negative charge will… attract An electron and a proton will… attract An electron and a neutron will… do nothing! Because neutrons don’t have charge. 3. How can you make something negatively charged? Add extra electrons to the object. 4. How can you make something positively charged? Take electrons away from the object. (Note: Protons cannot move without splitting the nucleus – this would cause an atomic explosion – NOT electric charge. Would be bad).