PFC2 Chapter 10 Section 2 Guided Reading What is a spectrum? A
advertisement

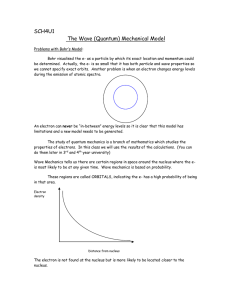
PFC2 Chapter 10 Section 2 Guided Reading 1. What is a spectrum? 2. A ________________ spreads light into its own individual colors. 3. Different colors of light have different amounts of _________________. 4. Danish physicist Neils Bohr proposed the concept of _________________ _________________ to explain the spectrum. 5. When an electron in an atom “jumps” from a higher energy level to a lower energy level, energy in the form of _________________ is released. 6. ______________ ______________ says that when a particle, such as an electron, is confined to a small space inside an atom, the energy, momentum, and other variables of the particle become quantized and can only have specific values. 7. The quantum model of the atom describes the electron as a “fuzzy” cloud of negative charge called a ______________ ______________ rather than as a particle moving around the nucleus. 8. State the Pauli exclusion principle. 9. What is Heisenberg’s “uncertainty principle?” 10. The smallest quantity of light energy is called a _________________. 11. Using Figure 10.14, explain what happens when you bounce a photon off an electron. 12. The science of describing the chance that an event or event will occur is called _________________. 13. Quantum theory uses probability to predict how likely it is that an electron will be found at various distances from a nucleus. This probability is described mathematically by a _________________ function.