SNC 1D Development of the Atomic Theory Match each name with
SNC 1D
Development of the Atomic Theory
Match each name with the appropriate description below. Each name may be used more than once.
(a) Aristotle
(b) Niels Bohr
(c) James Chadwick neutron
(d) John Dalton
(e) Democritus
(f ) Dmitri Mendeleev
(g) Ernest Rutherford
(h) J.J. Thomson
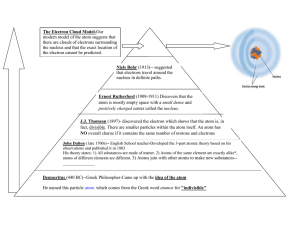
1. determined that the mass of a proton approximately equals the mass of a
2. discovery of the electron
3. discovery of the neutron
4. discovery of the proton
5. theory that an atom is mostly empty space
6. gold-foil experiment providing evidence of the nucleus in an atom
7. hypothesis (in the 1800s) that all matter is made up of indivisible atoms
8. organization of elements in the periodic table according to their properties and atomic numbers
9. prediction of the properties of elements unknown at that time
10. hypothesis that all atoms of an element are identical
11. idea that an atom is a positively charged sphere, with electrons evenly distributed throughout it, like the raisins in a plum pudding
12. hypothesis that atoms of different elements are different
13. demonstrated that electrons move around the nucleus in fixed orbits
14. stated that the nucleus of an atom is surrounded by a cloud of electrons
15. demonstrated that the nucleus of an atom contains most of its mass and is positively charged
16. theory of an “indivisible” atom electrons
17. statement that a neutral atom has the same number of protons and
18. hypothesis that matter is conserved during a chemical reaction
19. statement that the distance of an electron from the nucleus depends on the energy of the electron
20. claim that all matter is made from earth, water, air, and fire
21. theory that each electron orbit can hold a maximum number of electrons