TANUJ STRUCTURE OF ATOM
advertisement

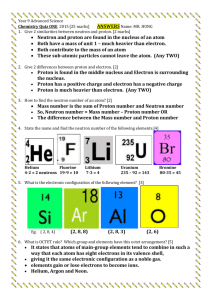
PROJECT NAME STRUCTURE OF ATOM NAME: TANUJ SAINI CLASS: 9 TH A ROLL NO.: 26 SUBMITTED BY : TANUJ SAINI SUBMITTED TO : MRS. GUNJAN GOYAL We will see many thing chair, table, wood ice in our daily life. Any thing which occupies space and has mass is called matter. All the matter is made up of tiny particles and particles are made up of Atom. Atom is the smallest part of the matter. Structure of Atoms:Atom is the smallest part of matter-An Atom is the smallest particle of an element. Atom is found impure stage. Nucleus Nucleus is the main part of the atom. Nucleus is made up of two particles 1) Protons 2) Neutrons The existence of protons in the atoms was shown by E. Goldstein. The proton is a positively charged particle found in the atoms of all the element. Characteristics of a Proton The two important characteristics of a proton are its mass and charge. These are described below. 1.Mass of a Proton The relative mass of a proton is 1u The absolute mass of a proton is 1.6x 10.24 gram. 2. Charge of a Proton So the absolute charge of a proton is 1.6×10*-19 coulomb of positive charge. The relative charge of a proton is +1 The Discovery of Neutron particle by chad wick in 1932. The neutron is a neutral particle found in the nucleus of an atom. The sub atomic particle not present in a hydrogen atom is neutron. Characteristics of a Neutron The two important characteristics of a neutron are its mass and charge. These are described below. 1. Mass of a Neutron The relative mass of a neutron is 1u. The absolute mass of a neutron is 1.6×10*-24 gram. 2. Charge of a Neutron Neutron has no charge. It is electrically neutral EX. Atomic mass of carbon= mass of 6 proton+ mass of 6 neutron =6×1+6×1 =12u The existence of electrons in an atom was shown by J. J. Thomson in 1897. Cathode rays consist of small, negatively charged particles called electrons. The electron is a negatively charged particle found in the atoms of all the element. Characteristics of an electron The two important characteristics of an electron are its mass and charge. These are described below. 1. Mass of an electron The relative mass of an electron is 1/1840 u the absolute mass of an electron is , how ever 9 ×10*-28 gram. 2. Charge of an electron The absolute charge on an electron is 1.6 ×10*-19 Coulomb of negative charge. The relative charge of an electron is,1