ch 7 FIB Key Terms.doc
advertisement

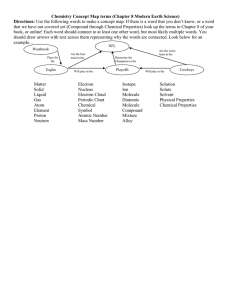
Name: _______________________________________ Date: __________________ Pd. Chapter 7 Key Terms - Fill in the blank with the correct key term reactant product Decomposition reaction oxidationreduction reaction Synthesis reaction free radical chemical equilibrium endothermic reaction Single displacement rx exothermic reaction Double displacement rx mole ratio catalyst substrate chemical energy Combustion reaction chemical equation enzyme Section 7.1 Nature of Chemical Reactions(Rx) 1. 2. 3. 4. 5. __________________ a substance or molecule that participates in a chemical reaction __________________ chemical reaction in which energy is absorbed __________________ chemical reaction in which energy is released __________________ the substance that forms in a chemical reaction __________________ the energy released when a chemical compound reacts to produce new compounds Section 7.3 Reaction Types 6. __________________ a reaction in which two or more substances combine to form a new compound; A + B -> AB 7. __________________ type of reaction a single compound breaks down to from two or more simpler substances; AB -> A + B 8. __________________ a reaction in which one element or radical takes the place or another element or radical in a compound; AB + C -> AC + B 9. __________________ a reaction in which a gas, a solid precipitate, or a molecule compound forms from the apparent exchange of atoms or ions between two compounds; AB + CD -> AD + CB 10. __________________ any chemical change in which one substance is oxidized(loses) and one substance is reduced(gains), also called redox reaction; Rust Fe loses and O gains, any Ionic bond results from losing and gaining of electrons 11. __________________ the rapid oxidation reaction of an organic compound, in which heat is released; oxygen is needed for fire 12. __________________ an atom or group of atoms that has one unpaired electron; forms covalent bonds Section 7.2 Chemical Equations 13. __________________ the relative number of moles of the substance required to produced a given amount of product in a chemical reaction 14. __________________ a representation of a chemical reaction that uses symbols to show the relationships between the reactants and the products Section 7.4 Reaction Rates and Equilibrium 15. __________________ a substance that changes the rate of a chemical reaction without being consumed or changed significantly; usually speeds up the rate 16. __________________ a molecule, usually a protein, that acts as a biological catalyst 17. __________________ the reactant (substance changed) in chemical reactions catalyzed by enzymes 18. __________________ state of balance in which the rate of the forward reaction balances the rate of the reverse reaction; uses a symbol Name the 7 Diatomic Molecules (Magic Seven) symbol with subscript