TEST #4 Blank
advertisement
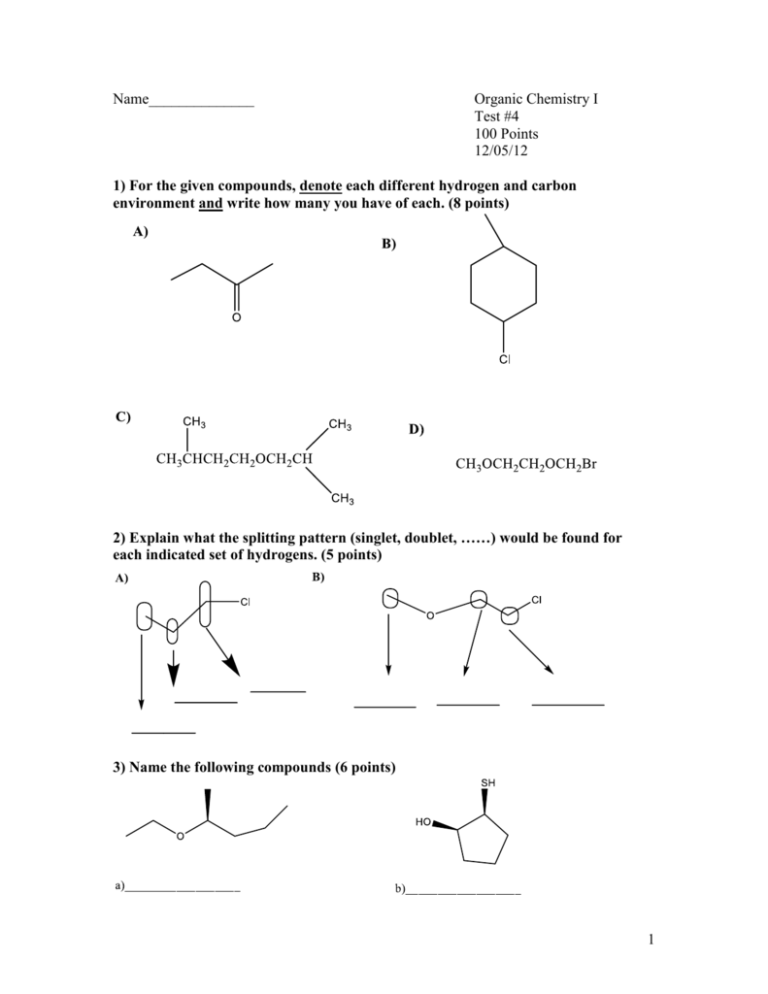
Name______________ Organic Chemistry I Test #4 100 Points 12/05/12 1) For the given compounds, denote each different hydrogen and carbon environment and write how many you have of each. (8 points) 2) Explain what the splitting pattern (singlet, doublet, ……) would be found for each indicated set of hydrogens. (5 points) 3) Name the following compounds (6 points) 1 4) For the following compounds below: (20 points) i) Draw a 1H-NMR: include relative shifts, splitting patterns (you should also assign each peak to the appropriate hydrogens of the compound, use arrows or labels to do this) ii) Draw a 13C-NMR: include relative shifts. (you should also assign each peak to the appropriate carbon of the compound, use arrows or labels to do this) a) 2-Chloro-2,3 Dimethyl butane 2 b) 3 5) From the given spectra and data. Identify the compound by drawing its structure. (20 points) [Make sure you write down all your work to get partial credit and you circle your final structure] a) 4 b) Molecular Formula: C5H9OBr H-NMR Data: C-NMR Data 5 6) What is the product and the mechanism for the following reaction? (6 points) 7) Design a synthesis of compound A from compound B. You can use any other reagents you like. (6 points) 6 8) Fill-in the reactions with the appropriate reagent, product or starting material (24 Points) 7 9) Dr. S. Black wanted to synthesize a new ester for his cologne “Nimbus”. The ester is seen below. Draw the structure of the carboxylic acid and the alcohol Dr. Black will need to make this ester. (5 points) O O 8











