2nd Semester Chemistry Final Review Worksheet
advertisement

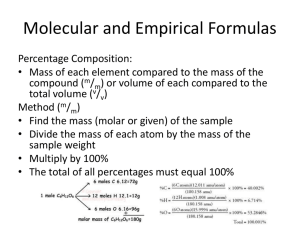
2nd Semester Chemistry Final Review Worksheet 1. Determine the empirical formula for a compound that is made up of: H= 9.15% C = 54.53% O = 36.32%. 2. Determine the molecular formula of the compound in #1 if the molecular mass of this compound is132.16. 3. Describe the relationship between gas volume and pressure using an example and explanation that an upper elementary student would understand. _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ 4. What law applies to the previous question? ____________________________________________________________ 5. What is the percent composition of each of the compounds in Copper (II) Hydroxide. 6. You worked for a month on a lab. Your math indicated that you would create 14.6g of Na2CO3. Instead, you got 12.9g. What was your percent yield? 7. What was your percent error in the lab in #6? 8. An aerosol can at room temperature (23oC) holds a volume of 850ml of gas under 220kPa of pressure. If you throw the can into a fire, the temperature increases to 900oC. What will the volume of the gas become? (may not get to) 9. How many grams of solid are needed to make 965ml of a 0.88M K3(PO4) solution? 10. Use the kinetic theory of matter to explain what absolute zero is. ______________________________________________________________________________________________ ______________________________________________________________________________________________ ______________________________________________________________________________________________ 11. What is the molarity of a solution that was made by dissolving 67.5g of Manganese Dioxide in 1.3L of water? Use the following information to answer questions 12 - 15 25.0 g of Iron reacts with 25.0g f Hydrochloric acid 12. Write a balanced equation for the reaction. 13. Determine the limiting reactant in the reaction. 14. How much of the excess reactant will remain when the reaction is completed? 15. Based on your work, how much Iron (II) Chloride can be made?