Ch3 * Carbonyl compounds:
advertisement
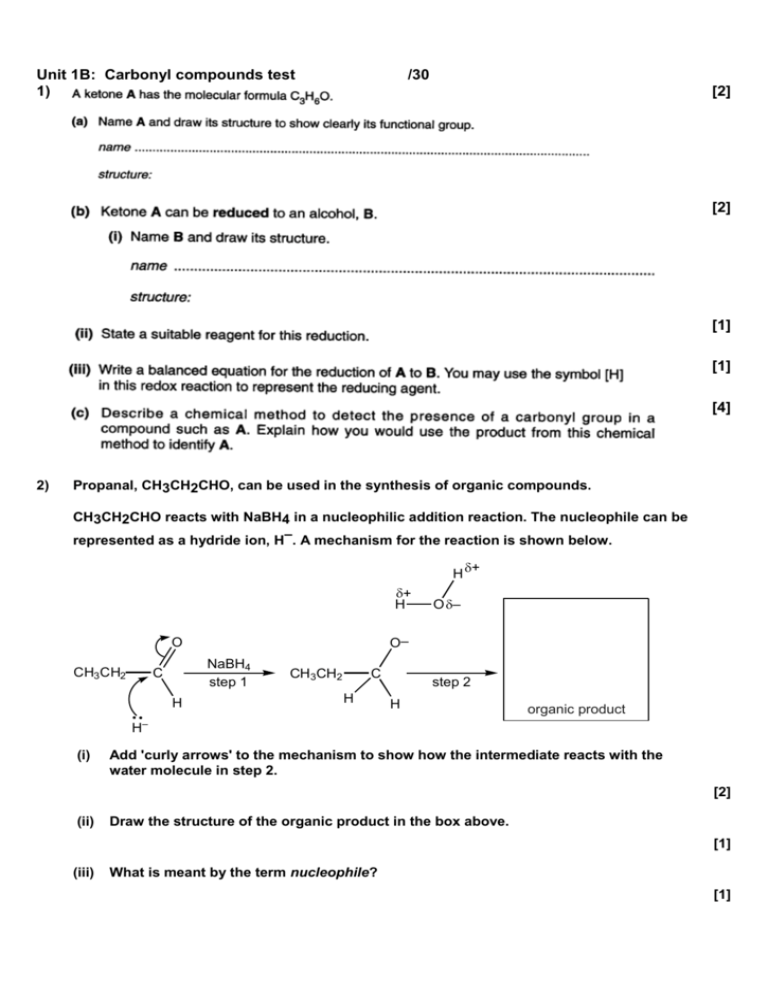
Unit 1B: Carbonyl compounds test 1) /30 [2] [2] [1] [1] [4] 2) Propanal, CH3CH2CHO, can be used in the synthesis of organic compounds. CH3CH2CHO reacts with NaBH4 in a nucleophilic addition reaction. The nucleophile can be represented as a hydride ion, H–. A mechanism for the reaction is shown below. H + H NaBH4 step 1 C H H (i) O – O– O CH3C H2 + CH3CH2 C H step 2 H organic product – Add 'curly arrows' to the mechanism to show how the intermediate reacts with the water molecule in step 2. [2] (ii) Draw the structure of the organic product in the box above. [1] (iii) What is meant by the term nucleophile? [1] (iv) 3) Describe, in words, exactly what is happening to the electron pairs and bonds in step 1 of the mechanism above. [3] Glyceryl trihexanoate is a triglyceride that can be made from glycerol (propane-1,2,3-triol) and hexanoic acid, C5H11COOH. a) Draw the structure of glyceryl trihexanoate. Show every bond in the functional groups. [2] b) Naturally occurring glyceryl tritrihexanoate is found in vegetable oil. It can be used to produce biodiesel using transesterification. Write a chemical equation outlining the reaction, giving all reagents necessary. [3] 4) As a wine ages, some of the carboxylic acids slowly react with ethanol in the wine to produce esters. (i) Draw a displayed formula to show the structure of the ester formed when propanoic acid reacts with ethanol. [1] (ii) Suggest what effect this process might have on the flavour of the wine. Explain your reasoning. [1] 5) Compound A is used to add the flavour of mushrooms to foods. O C H 3C C H CH2 O C CH3 compound A (a) (i) Apart from the benzene ring, name the two functional groups in compound A. [2] (ii) Draw the skeletal formula of compound A. [1] (iii) Deduce the molecular formula of compound A. [1] (b) If the food is cooked for a long time, naturally occurring acids catalyse the hydrolysis of compound A. Draw structures to show the two organic compounds formed by the acid hydrolysis of compound A. [2]











