Atomic Notation Worksheet: Chemistry Practice
advertisement
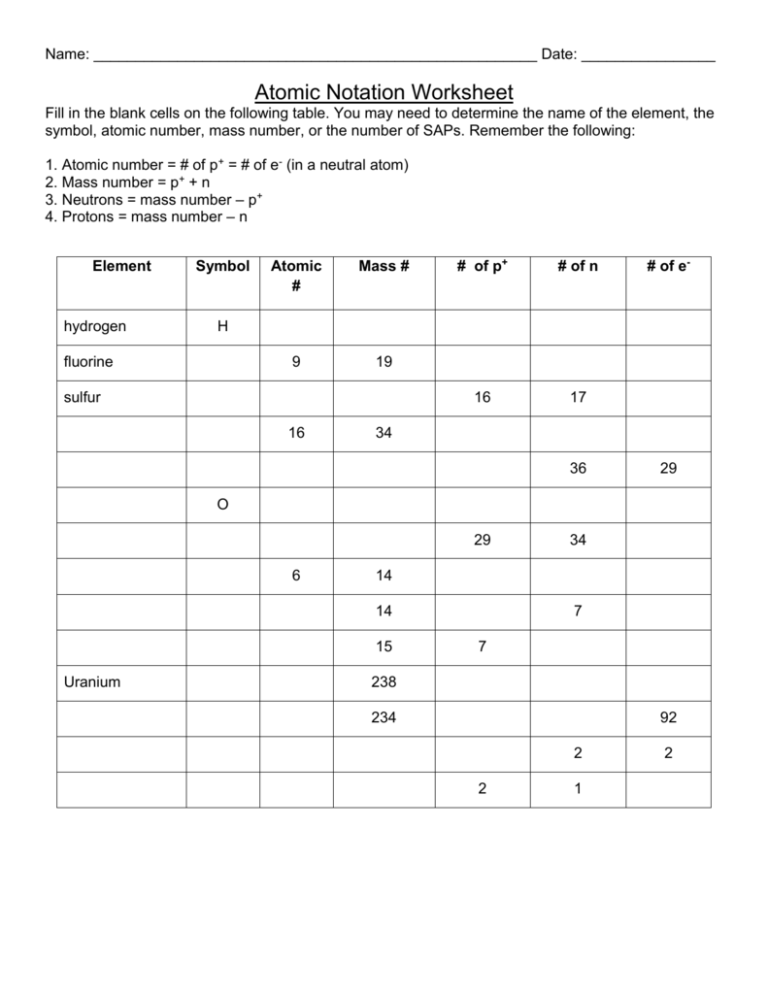
Name: _____________________________________________________ Date: ________________ Atomic Notation Worksheet Fill in the blank cells on the following table. You may need to determine the name of the element, the symbol, atomic number, mass number, or the number of SAPs. Remember the following: 1. Atomic number = # of p+ = # of e- (in a neutral atom) 2. Mass number = p+ + n 3. Neutrons = mass number – p+ 4. Protons = mass number – n Element hydrogen Symbol Atomic # Mass # 9 19 # of p+ # of n 16 17 # of e- H fluorine sulfur 16 34 36 29 O 29 6 14 14 15 Uranium 34 7 7 238 234 92 2 2 1 2 1. Give the symbol and number of protons in one atom of: Lithium __________________ Bromine __________________ Iron __________________ Copper __________________ Oxygen __________________ Mercury ________________ Krypton __________________ Helium __________________ Tin ____________________ 2. Give the symbol and number of electrons in a neutral atom of: Uranium __________________ Chlorine __________________ Boron __________________ Iodine __________________ Antimony ________________ Xenon _________________ 3. Give the symbol and number of neutrons in one atom of the element below. To get “mass number” you must round the “atomic mass” to the nearest whole number. Show your calculations. Barium __________________ Bismuth __________________ Carbon ________________ Hydrogen ________________ Fluorine __________________ Magnesium _____________ Europium __________________ Mercury __________________ Radium ________________ 4. Name the element which has the following numbers of particles: a. 26 electrons, 29 neutrons, 26 protons _____________________ b. 53 protons, 74 neutrons _____________________ c. 2 electrons (neutral atoms) _____________________ d. 20 protons _____________________ e. 86 electrons, 125 neutrons, 82 protons (charged atom) _____________________ f. 0 neutrons _____________________ 5. If you know only the following information can you always determine what the element is? Yes or No. a. number of protons ___________ b. number of neutrons___________ c. number of electrons in a neutral atom___________ d. number of electrons___________











