Atomic Number and Mass Number
advertisement

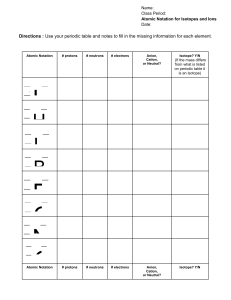
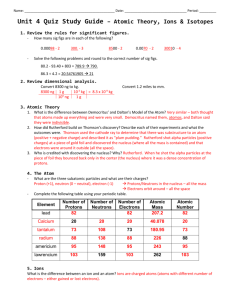
Name: ________________________________ Period: ________________ Date: ___________ Atomic Number – This number represents the number of protons in an element. Atomic Mass – This number represents the total number of protons and neutrons in the element. This is an average of all isotope masses. Cation – An element whose number of electrons is less than the number of protons. It has lost electrons. Anion - An element whose number of electrons is more than the number of protons. It has gained electrons. Isotope – An element that has a different number of neutrons in the nucleus giving it a different mass Nuclear Format Mass number Atomic Number Ionic Format 235 92𝑈 𝐴𝑙 +3 Charge on ion Complete the following chart and answer the questions on back. Write the element symbol in the correct format (nuclear or ionic) for the given information. In the final column, identify the element as common (as seen on the table), an isotope, or an ion. Element Name Atomic Number Number of Protons Number of Neutrons Number of Electrons Mass Number 235 92𝑈 𝑉 +5 H 6 14 𝐼−1 64 38 90 15 7𝑁 109 42 37 18 80 W 9 10 Ho 106 𝐴𝑔+1 95 * 112 *New symbol 89 241 Identity How are the atomic number and the number of protons related to each other? How do the number of protons, number of neutrons, and the mass number relate to each other? What is the one thing that determines the identity of an atom (that is, whether it is an oxygen atom or a carbon atom, etc.)? Why is a cation positive? Why is an anion negative? What element would you get if you combined the following atoms? Write the equation as A + B → C in nuclear format. a) Rhenium and manganese b) Sodium and yttrium c) Curium and hydrogen As these products stable (common) atoms or isotopes? What can you conclude about combining atoms?