Atomic Structure Activity
advertisement

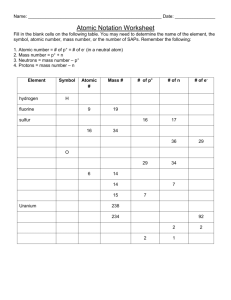
Atomic structure Name: _______________________________________ 1. What does the word neutral mean? ________________________________________________ Give an example of something that is neutral: ____________________________________________ 2. What does it mean to be electrically neutral? _________________________________________ Give an example of something that is electrically neutral: ___________________________________ Facts: a) Atoms are electrically neutral b) Protons are positive c) Electrons are negative 3. What must a neutral atom contain and why? 4. Draw a neutral atom : Fact: The number of protons identifies the element 5. What element did you create? How do you know? 6. What is meant by mass? Fact: The mass of an atom is made up of protons and neutrons. 7. Create an atom with a mass of 14. What atom did you create and how do you know? Fact: Only protons and neutrons are in the nucleus. 8. What are two more facts that you can state about electrons? Facts: 1) 2) 18.998 Atomic mass F Atomic symbol 9 Atomic number 1) 2) 3) 4) Rounds to mass # = protons + neutrons = number of protons What is the mass number for fluorine? ______ How many protons does fluorine have? ______ How many neutrons does fluorine have? _____ How many electrons does fluorine have? _____ Build a fluorine atom using the paper plate as the nucleus – copy it below. Fact: Isotopes are neutral atoms that have the same atomic number but a different number of neutrons. 1. Build two isotopes of fluorine – what is the only difference between the two? 2. Carbon-14 (146C) is a radioactive isotope that is commonly found in living beings. It is Carbon with a mass number of 14. Build a carbon-14 atom – identify the number of protons, neutrons and electrons – put your information below. #protons = _____ #neutrons= _____ # electrons = _____ Facts: 1) Ions are not neutral 2) Cations are positive ions 3) Anions are negative ions 1. Build a phosphorus ion that has a negative three charge : P3-