Chemistry Study Guide: Atoms, Elements, and Changes
advertisement
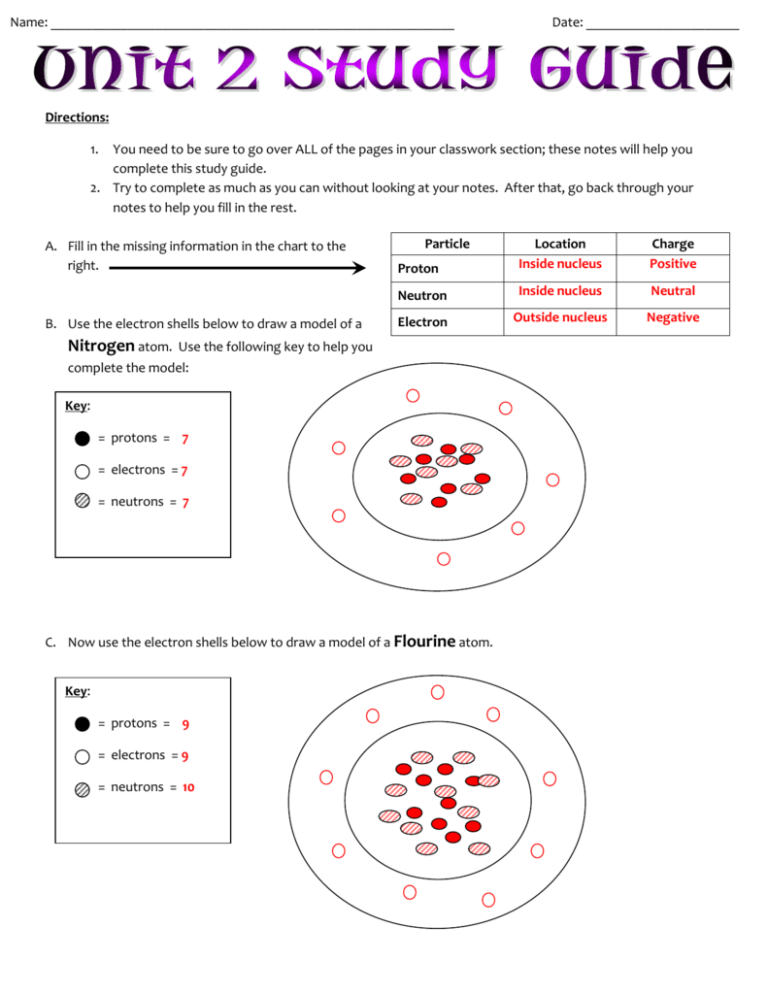
Name: __________________________________________________________ Date: ______________________ Directions: 1. You need to be sure to go over ALL of the pages in your classwork section; these notes will help you complete this study guide. 2. Try to complete as much as you can without looking at your notes. After that, go back through your notes to help you fill in the rest. A. Fill in the missing information in the chart to the right. B. Use the electron shells below to draw a model of a Nitrogen atom. Particle Proton Location Inside nucleus Charge Positive Neutron Inside nucleus Neutral Electron Outside nucleus Negative Use the following key to help you complete the model: Key: = protons = 7 = electrons = 7 = neutrons = 7 C. Now use the electron shells below to draw a model of a Flourine atom. Key: = protons = 9 = electrons = 9 = neutrons = 10 a. Circle the part of the atom that makes these two elements VERY different from each other? PROTONS NEUTRONS ELECTRONS D. Use your periodic table to fill in the missing information below. This is the ATOMIC NUMBER. The atomic number tells us the number of This is the PROTONS and ELECTRONS Chemical SYMBOL This is the This is the ATOMIC MASS. This tells us the number of protons and neutrons in the atom. Element NAME E. Use your periodic table to fill in the missing information below. Chemical Name Chemical Symbol Atomic Number Atomic Mass Protons Electrons Neutrons Potassium K 19 39 19 19 20 NICKEL Ni 28 59 28 28 31 NEON Ne 10 20 10 10 10 F. A chemical formula is a way of writing a compound to tell us what ELEMENTS are in the compound. a. Look at the following chemical formulas and identify what ELEMENTS are in the compound. b. Then, tell us how many ATOMS of each element are present in the compound. N2O H2SO4 C8H10N4O2 Nitrogen # of Atoms 2 Hydrogen # of Atoms 2 Oxygen 1 Sulfur Oxygen Elements Elements Carbon # of Atoms 8 1 Hydrogen 10 4 Nitrogen 4 Oxygen 2 Elements G. Silver Nitrate is one of the compounds we used in our lab. a. Silver Nitrate has the following elements in it, in this exact order: i. One atom of Silver (Ag) ii. One atom of Nitrogen (N) iii. Three atoms of Oxygen (O) b. Use the above information to write the CHEMICAL FORMULA: AgNO3 H. Using the idea bank below, fill in the following venn diagram with information about physical and chemical changes. Physical Changes Chemical Changes True of Both B A H E C G D F IDEA BANK A. A gas is produced E. Change in size B. Change in shape F. Can see bubbling occur C. Cannot be easily reversed G. Change in state D. New substance is formed H. Change in matter I. A chemical equation is a way to represent a chemical change. a. Using the chemical equation below, DRAW A CIRCLE DRAW A SQUARE around the PRODUCTS. Zn + HCl around the REACTANTS and ZnCl2 + H2 J. Use the chart below to fill in the top 3 elements found in each of these locations. Use the graphs/charts below to help you fill in the missing information. Earth’s Atmosphere Earth’s Crust Human Body Earth’s Ocean Top Element # 1 Nitrogen Oxygen Oxygen Oxygen Top Element # 2 Oxygen Silicon Carbon Hydrogen Top Element # 3 Argon Aluminum Hydrogen Chlorine











