Atomic Symbol Worksheet
advertisement

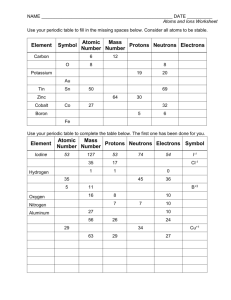
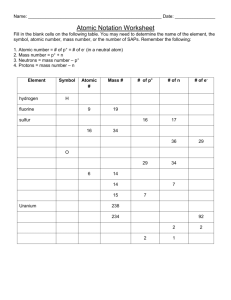
Name: _______________________________ Atomic Symbol Worksheet *Atomic number is always the same as number of protons. *# of protons = # of electrons unless you have an ION (+ or – charged atom such as Cu+2). If ion is negative, then electrons were gained. If ion is positive, electrons are lost. The magnitude of the charge indicates the number of electrons gained or lost. *Mass number (atomic mass rounded off) = protons + neutrons *When a symbol in a problem is written as: 14 6 C , the top number is the mass number and the bottom is the atomic number. *When atoms of the same element have different numbers of neutrons, we call them ISOTOPES. Fill in missing information: Complete the following table. Symbol 14 7 Name Atomic Number Atomic Mass # of Protons # of Neutrons # of Electrons Overall Charge N Neon 137 56 Ba 79 Lead 20 13 10 13 13 94 1 0 1 1 1 1 1 2 1 Uranium 24 12 Mg 7 3 Li 80 35 +2 +1 Br −1 Additional Nuclear Symbols Another symbol for elements includes: Carbon – 12. The elements name is given instead of the symbol and 12 represents the mass number of the element. Symbol Carbon – 14 Protons Neutrons 53 127 Electrons Strontium – 90 Uranium - 235 Boron - 11 92 32 16 11 5 238