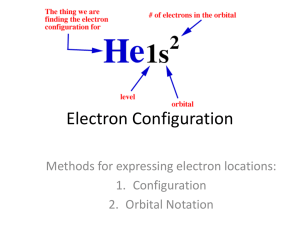
Electron-configurations
advertisement

Electron configurations 10/23/12 What is an electron configuration? • The ways in which the atoms are arranged in orbitals around the nucleus of an atom. What is an atomic orbital? • A region of space where an electron is likely to be found. orbital Describe the shape Draw a picture of the shape Write the number of orbitals at each energy level s Round, like a ball p d f Two raindrops connected 1-4 = clover leaf shape 5= raindrops with a ring around the middle 8 regions in different planes 1 3 5 7 What are the rules for filling orbital diagrams • Aufbau Principle: Electrons fill lowest energy levels first. • Pauli Exclusion Principle: Each orbital can have 2 electrons. They must have opposite spin. • Hund’s Rule: Electrons will fill in the same energy level, so that the number of electrons with the same spin is as great as possible. How do you complete an orbital filling diagram? How do you write out an electron configuration? • Standard method: 1s2 2s2 2p6 3s2 3p6 4s1 • Short hand method: [Ar] 4s1 What are Valence Electrons? • Electrons in the highest occupied energy level of an atom. • These are the electrons that largely determine the What is the octet rule? • In forming bonds or reacting with other elements, an atom will usually try to have 8 valence electrons.