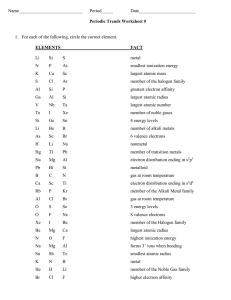
Atomic Structure Essay Question
advertisement
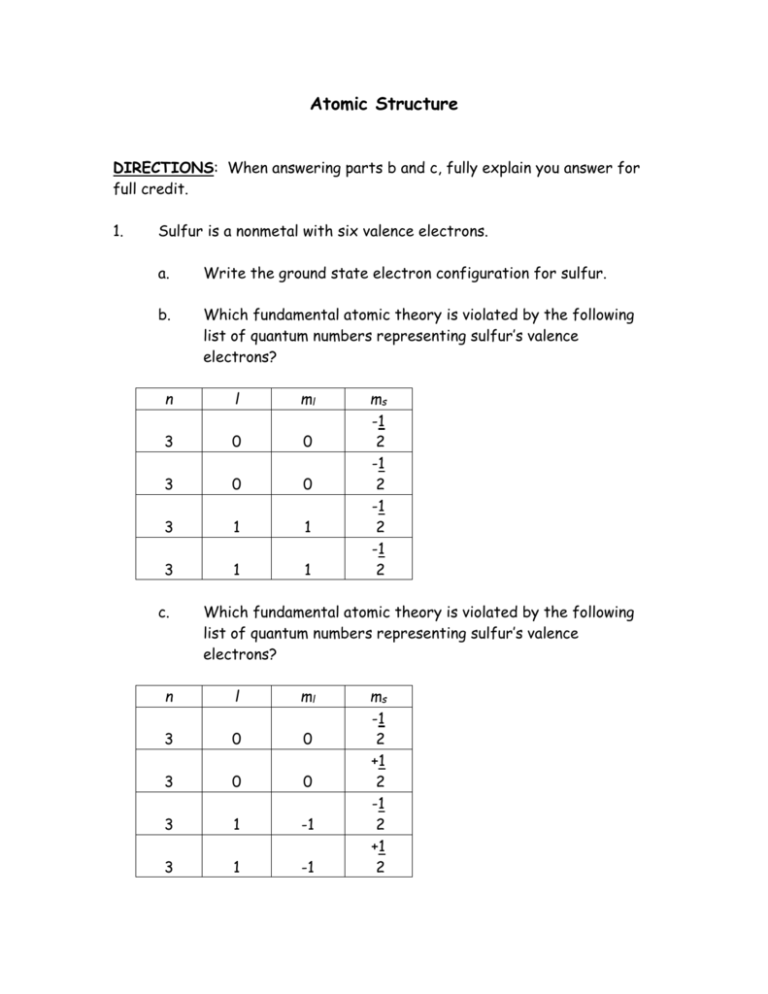
Atomic Structure DIRECTIONS: When answering parts b and c, fully explain you answer for full credit. 1. Sulfur is a nonmetal with six valence electrons. a. Write the ground state electron configuration for sulfur. b. Which fundamental atomic theory is violated by the following list of quantum numbers representing sulfur’s valence electrons? n l ml 3 0 0 3 0 0 3 1 1 3 1 1 c. ms -1 2 -1 2 -1 2 -1 2 Which fundamental atomic theory is violated by the following list of quantum numbers representing sulfur’s valence electrons? n l ml 3 0 0 3 0 0 3 1 -1 3 1 -1 ms -1 2 +1 2 -1 2 +1 2 2. For the element antimony, list a possible set of values for the four quantum numbers of all the valence electrons if each is in its ground state. 3. Identify the following elements: a. An excited state of this element has the electron configuration 1s22s22p53s1 b. The ground-state electron configuration is [Ne]3s23p4 c. An excited state of this element has the electron configuration [Kr]5s24d65p26s1 d. The ground-state electron configuration contains three unpaired 6p electrons.