Sample Exam Solutions
advertisement
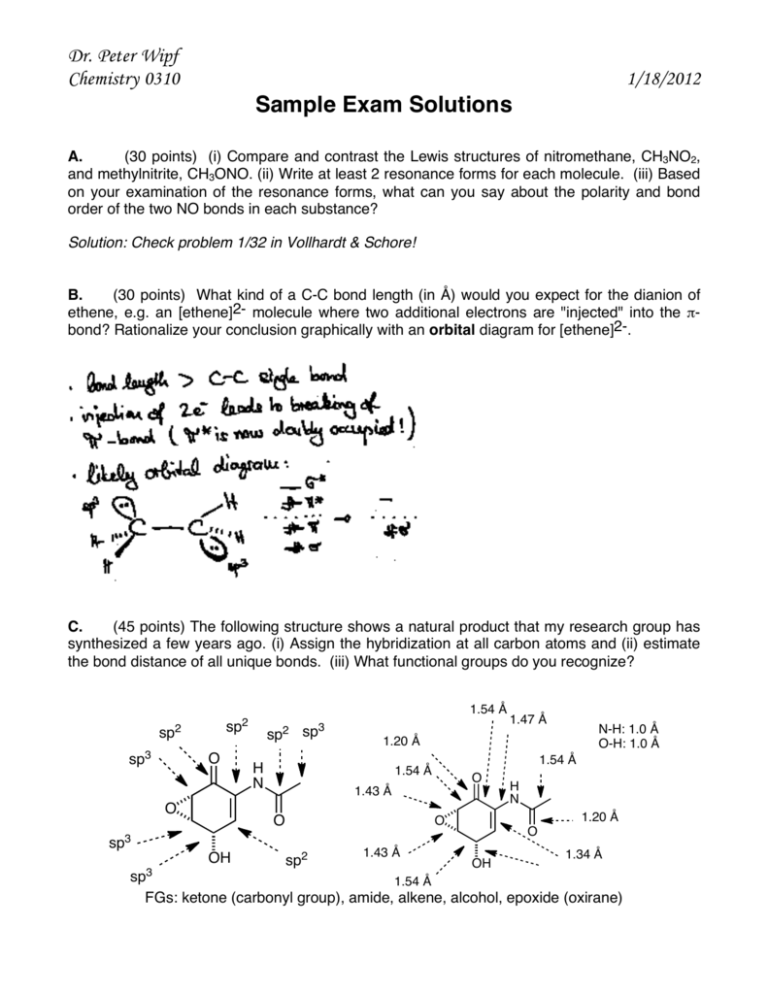
Dr. Peter Wipf Chemistry 0310 1/18/2012 Sample Exam Solutions A. (30 points) (i) Compare and contrast the Lewis structures of nitromethane, CH3NO2, and methylnitrite, CH3ONO. (ii) Write at least 2 resonance forms for each molecule. (iii) Based on your examination of the resonance forms, what can you say about the polarity and bond order of the two NO bonds in each substance? Solution: Check problem 1/32 in Vollhardt & Schore! B. (30 points) What kind of a C-C bond length (in Å) would you expect for the dianion of ethene, e.g. an [ethene]2- molecule where two additional electrons are "injected" into the πbond? Rationalize your conclusion graphically with an orbital diagram for [ethene]2-. C. (45 points) The following structure shows a natural product that my research group has synthesized a few years ago. (i) Assign the hybridization at all carbon atoms and (ii) estimate the bond distance of all unique bonds. (iii) What functional groups do you recognize? 1.54 Å sp2 sp2 sp3 O O H N OH N-H: 1.0 Å O-H: 1.0 Å 1.54 Å 1.54 Å O 1.43 Å 1.43 Å H N 1.20 Å O sp2 1.47 Å 1.20 Å O sp3 sp3 sp2 sp3 O OH 1.34 Å 1.54 Å FGs: ketone (carbonyl group), amide, alkene, alcohol, epoxide (oxirane) CHEM 0310 D. Page 2 (30 points) Which of the following structures violate the octet rule? H H N H H O O Cl O H C H H C C H H H Br P Br Br H H H H H E. (40 points) Draw a Newman projection of the gauche conformation of butane and explain the reasons for its energetic destabilization (by how much?) vs. the antiperiplaner conformation. The gauche interaction suffers from electron (orbital-orbital electron repulsion) as well as steric destabilization due to the interaction of the two large methyl groups which are further apart in the antiperiplanar orientation. The energy destabilization is about 0.9 kcal/mol. F. (45 points) (i) Determine whether each species in the following equations is acting as a Brønsted acid or base, and label it. (ii) Indicate whether the equilibrium lies to the left or the right. (iii) Estimate K for each equation using the Table copied on the next page. Solution: Check problem 2/27 in Vollhardt & Schore! CHEM 0310 G. Page 3 (30 points) Use orbitals to explain the hyperconjugative stabilization of the ethyl radical. hyperconjugation is the electron donation of a filled sp3 orbital of a neighboring C-H bond into the partially filled p-orbital of the primary radical in the ethyl group. H. (50 points) Write in full the mechanism for monobromination of methane. Clearly indicate initiation, propagation, and termination steps.