Worksheet 23 (Chapter 9.6-9.8)
advertisement
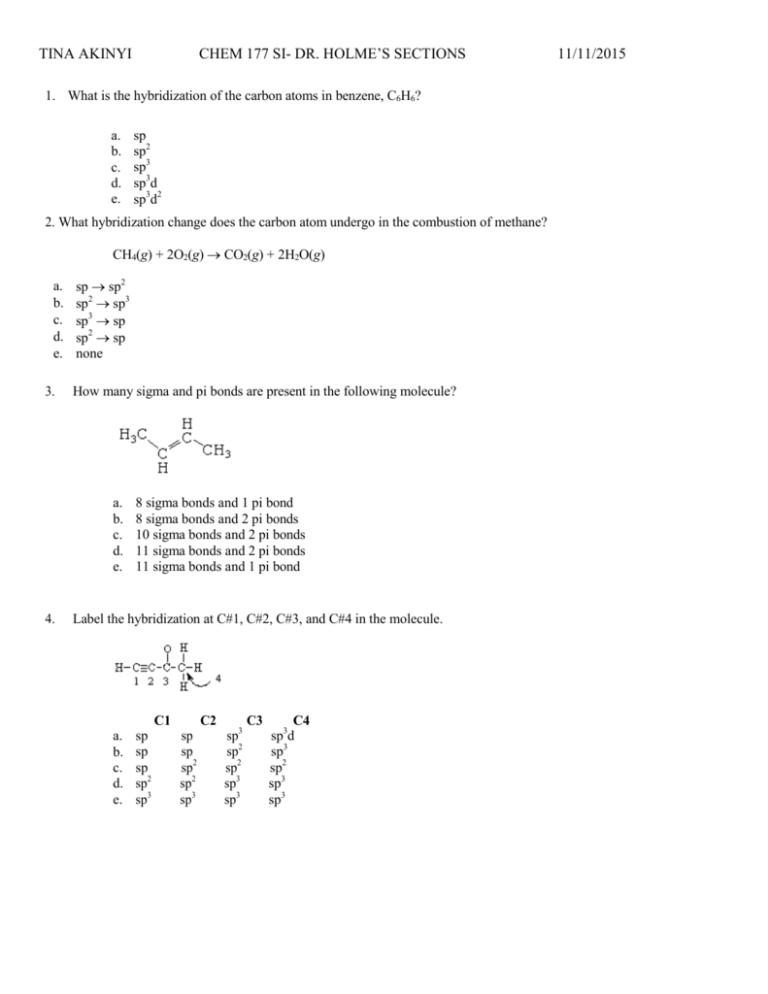
CHEM 177 SI- DR. HOLME’S SECTIONS TINA AKINYI 1. What is the hybridization of the carbon atoms in benzene, C6H6? a. b. c. d. e. sp sp2 sp3 sp3d sp3d2 2. What hybridization change does the carbon atom undergo in the combustion of methane? CH4(g) + 2O2(g) CO2(g) + 2H2O(g) a. b. c. d. e. 3. sp sp2 sp2 sp3 sp3 sp sp2 sp none How many sigma and pi bonds are present in the following molecule? a. b. c. d. e. 4. 8 sigma bonds and 1 pi bond 8 sigma bonds and 2 pi bonds 10 sigma bonds and 2 pi bonds 11 sigma bonds and 2 pi bonds 11 sigma bonds and 1 pi bond Label the hybridization at C#1, C#2, C#3, and C#4 in the molecule. C1 a. b. c. d. e. sp sp sp sp2 sp3 C2 sp sp sp2 sp2 sp3 sp3 sp2 sp2 sp3 sp3 C3 C4 sp3d sp3 sp2 sp3 sp3 11/11/2015 TINA AKINYI CHEM 177 SI- DR. HOLME’S SECTIONS 5. How many sigma () bonds and pi () bonds are in the following molecule? a. b. c. d. e. 6. five and two five and three five and five seven and two seven and three Which of the following characteristics apply to PCl3? 1. 2. 3. 4. nonpolar molecule polar bonds trigonal-pyramidal molecular geometry sp2 hybridized a. b. c. d. e. 1 and 2 2 and 3 3 and 4 1, 2, and 3 1, 2, 3, and 4 7. Which of the underlined atoms (C1, C2, N, and O) are sp2 hybridized? a. b. c. d. e. C1 and C2 C1, N, and O N and O O and C2 O only 11/11/2015 CHEM 177 SI- DR. HOLME’S SECTIONS TINA AKINYI 11/11/2015 Molecular Orbital Theory The following molecular orbital diagram may be used for the following problems. For oxygen and fluorine, the 2p orbital should be lower in energy than the 2p. However, the diagram will still yield correct bond order and magnetic behavior for these molecules. 8. According to molecular orbital theory, which of the following species is the most likely to exist? a. b. c. d. H22He2 Li2 Be2 *We will discuss bonding/antibonding in tomorrow’s SI session* TINA AKINYI CHEM 177 SI- DR. HOLME’S SECTIONS 11/11/2015 TINA AKINYI CHEM 177 SI- DR. HOLME’S SECTIONS 11/11/2015