Avogadro*s Constant and the Mole
advertisement

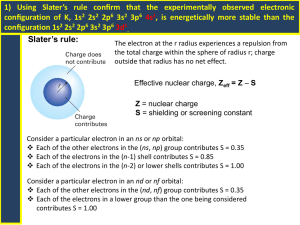
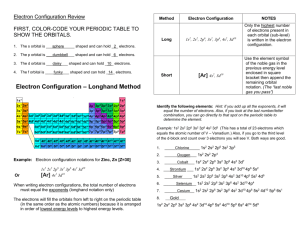
2 ATOMIC STRUCTURE 2.2 ELECTRON CONFIGURATION 2.2.1 The arrangement of electrons in atoms Electrons → exist outside of the nucleus of an atom → are kept from leaving by the electrostatic force of attraction between the positively charged nucleus and the negatively charged electron. → do not exist in certain areas and are more likely to exist in others because of quantum theory – an electron behaves as both a particle and a wave! The Periodic Table of the Elements is a remarkable model for chemical behaviour and quantum behaviour of electrons. Evidence for electron energy levels in atoms Spectroscopy the study of the absorption and emission of electromagnetic radiation by matter. A. Faunhofer Lines B. Flame Tests Fraunhofer (1850) – observed dark lines in the continuous spectrum of light from the Sun and bright stars like Sirius Bunsen and Kirchhoff (1859) – discovered that different metal ions produced distinctive colours when burned in a flame. http://www.aip.org/history/cosmology/tools/tools-spectroscopy.htm C. Emission Spectra Niels Bohr (1913) – explained the emission spectra of hydrogen in terms of electron “jumping” between energy levels. Continuous Spectrum → white light = all colours (ROYGBIV); produced by very hot objects (Sun, incandescent light, candle) Emission Spectrum → lines of bright colour produced by hot gases. Each element has a distinctive emission spectrum. Absorption Spectrum = reverse of emission spectrum; produced when white light passes through cold a gas Bohr Postulates 1. Circular Orbits Electrons exist in circular orbits about the nucleus. The centripetal force is an electrostatic attraction between the nucleus (+) and the electrons (−) 2. Energy Levels Electrons can only orbit on certain discrete energy levels. The energy levels are quantized (limited to a discrete set of values) 3. Electron Jumps An electron does not emit energy (light) when it stays in the same orbital (energy level). An electron can “jump” up to a higher energy level (excited state) if it absorbs energy equal to the difference in energy levels An “excited” electron can “jump down” to a lower energy level by releasing photons of energy equal to the difference in energy levels. Bohr related the difference in the electron energy levels (ΔE) to the energy of the photons of light emitted (hf), where h is Planck’s constant (h = 6.626 x 1036 J s) and f is the frequency of the electromagnetic radiation, according to: | Ef – Ei | = ΔE = hf Bohr determined the energy levels of hydrogen using the relationship where n = the principal quantum number (any integer representing the nth energy level. Spectrum Series → determined by the energy level to which the electrons fall (mnemonic: LUV VIS PASCHEN) LUV VIS PASCHEN |En – E1| |En – E2| |En – E3| Convergence Lyman series = Ultraviolet jump down to ground state (E1) Balmer series = Visible (ROYGBIV) jump down to E2 Paschen series = Infrared jump down to E3 highest ΔE small ΔE Within each spectral series, the spectral lines converge at higher frequencies (shorter wavelengths) Higher energy levels have smaller differences, so the size of the jumps down are similar (form a continuum) Limitations of the Bohr Model – the calculations are only valid for hydrogen spectra − the principles apply to the energy levels and spectra of all atoms Quantum Numbers principal quantum number n orbital quantum number ℓ magnetic quantum number mℓ spin quantum number s energy level of electron orbital shape within the energy level) orientation in an external magnetic field electron acts as a spinning charge producing its own magnetic field n = 1, 2, 3, . . . ℓ → s, p, d, f mℓ = +1 or 0 or −1 s = + ½ (up) or s = − ½ (down) Pauli Exclusion Principle → two electrons in the same atom cannot have the same four quantum numbers This principle helps to explain the periodic table → s-block, p-block, d-block and f-block, periods, and magnetic properties of some elements. 2.2.2 Full electron configurations Quantum numbers 1. Principle quantum number, n n = 1, 2, 3, … the number of the energy level and describes the relative size of the electron cloud 2. Sub-energy level Each energy level has as many sub-energy levels as the principle quantum number. This is also referred to as the orbital quantum number and refers to the shape of the electron cloud. Principle energy level Sub-levels n=1 s only (1s) n=2 s and p only (2s and 2p) n=3 s, p and d only (3s, 3, p, 3d) n=4 s, p, d, and f (4s, 4p, 4d, 4f) 3. Orientation describes the orientation of each orbital. There are three p orbitals that are orthogonal (perpendicular) to each other → px, py, pz Degenerate → same energy level All three 2p sub-levels have the same energy; they are degenerate 4. Spin Electrons have spin values that can be clockwise ( ) or anticlockwise ( ) Filling Energy Levels Aufbau principle Electrons fill sub-levels from the lowest energy upwards. NB 4s < 3d → Fill the 4s before 3d and fill 4f before 5d The entire electron configuration provides the lowest potential energy arrangement of the electrons. Pauli exclusion principle No two electrons in an atom can have the same set of quantum numbers → Each orbital can have a maximum of two electrons. Electron in the same orbital have opposite spins. Hund’s rule Electrons fill orbitals of the same energy to give the maximum number with the same spin. Representative Elements 1s2 2He Exceptions – half-fill or complete lower (similar) energy orbital 10Ne 1s2 2s2 2p6 = [He] 2s2 2p6 18Ar 1s2 2s2 2p6 3s2 3p6 = [Ne] 3s2 3p6 30Zn 1s2 2s2 2p6 3s2 3p6 4s2 3d10 = [Ar] 4s2 3d10 36Kr 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 = [Ar] 4s2 3d10 4p6 24Cr 1s2 2s2 2p6 3s2 3p6 4s1 3d5 = [Ar] 4s1 3d5 half full 29Cu 1s2 2s2 2p6 3s2 3p6 4s1 3d10 = [Ar] 4s1 3d10 3d full Energy Level Diagrams orbital diagrams showing individual electrons as arrows with spin indicated Electrons in boxes