Ch. 4 Worksheet Review Section 1 In order for an electron to be
advertisement

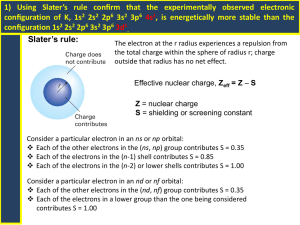
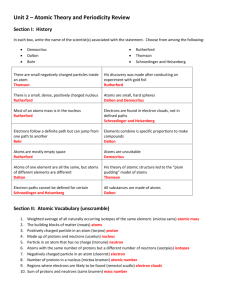
Ch. 4 Worksheet Review Section 1 1. 2. 3. 4. 5. 6. 7. 8. 9. In order for an electron to be ejected from a metal’s surface, the electron must be struck by a single photon with at least the minimum energy needed to knock the electron loose. The ground state is the lowest energy state of the atom. When the atom absorbs energy, it can move to a higher energy state, or excited state. A photon is emitted when an atom moves from an excited state to its ground state or to a lower-energy (but still excited) state. When an electron loses energy, it falls from a higher energy state to a lower energy state. The frequency of the emitted light, observed in an element’s line-emission spectrum, may be measured. The energy of each transition is calculated using the equation E=hv, where v is the frequency of each of the lines in the element’s line-emission spectrum. From the analysis of these results, the energy levels of an atom of each element may be determined. Energy is directly proportional to frequency, and ultraviolet radiation has a higher frequency than infrared radiation. Therefore, to produce ultraviolet radiation, electrons must drop to lower energy levels than they do to produce infrared radiation. Assuming both waves are travelling at the same speed, wave B would have the higher frequency. If speed is the same, then wavelength is inversely proportional to frequency, so as wavelength decreases, the frequency increases. Six. Each line represents the production of a single photon. The photon is produced as one electron falls from a higher energy level to a lower energy level. 9.7 × 1014 Hz 9.4 × 109 m Section 2 1. 8. d 2. a 3. a 4. c 5. c 6. c 7. c Scientists already had evidence that waves confined to a space could have only certain frequencies. De Broglie suggested that electrons should be considered as waves confined to the space around an atom’s nucleus. Therefore electron waves could only exist at certain frequencies. With the equation E=hv, the frequencies correspond to the quantum energies of the orbitals in the Bohr atomic model. 9. The principal quantum number refers to the energy level the electron is in. The angular momentum quantum number is the type of orbital which has a designated shape. The magnetic quantum number describes the orientation of an orbital around the nucleus. The spin quantum number gives the spin direction of an electron in an orbital. 10. The Heisenberg uncertainty principle states that it is impossible to determine simultaneously the position, velocity, and mass of an electron (or any other particle for that matter). There is always an uncertainty in trying to establish the location of an electron. Since the electron cannot be exactly located, the electron cloud/orbital represents the 3-D region where the electron is most likely located. 11. Fill in the table Principal Quantum Number (n) Number of Sublevels Types of Orbitals 1 1 s only 2 2 s&p 3 3 s, p, & d 4 4 s, p, d, & f Section 3 1. 2. The Pauli Exclusion Principle states that no two electrons in an atom may have the same set of four quantum numbers. If both electrons in the same orbital had the same spin state, each electron would have the same four quantum numbers. If one electron has the opposite spin state, the fourth quantum number is different and the principle is obeyed. This orbital notation is possible if the helium atom is in an excited state. (Although if the 2s electron fell back to the first energy level, it would violate the Pauli Exclusion Principle.) 3. P 1s2 2s2 2p6 3s2 3p3 P ____ 1s ____ 2s ____ ____ ____ 2p 4. N 1s2 2s2 2p3 N ____ 1s ____ 2s ____ ____ ____ 2p 5. K 1s2 2s2 2p6 3s2 3p6 4s1 K ____ 1s ____ 2s 6. Al 1s2 2s2 2p6 3s2 3p1 Al ____ 1s 7. Ar 1s2 2s2 2p6 3s2 3p6 Ar ____ 1s 8. B 9. ____ 3s ____ ____ ____ 3p ____ ____ ____ 2p ____ 3s ____ ____ ____ 3p ____ 2s ____ ____ ____ 2p ____ 3s ____ ____ ____ 3p ____ 2s ____ ____ ____ 2p ____ 3s ____ ____ ____ 3p ____ 4s 1s2 2s2 2p1 B ____ ____ ____ ____ ____ 1s 2s 2p Hund’s Rule or Pauli Exclusion Principle? a. Pauli Exclusion Principle: The 3s2 electrons have the same quantum numbers. (3, 0, 0, +½) b. Hund’s Rule: The 2p orbitals must each receive one electron with the same spin in each orbital, before pairing up. Mixed Review 1. 2. 3. A photon is emitted when an electron moves from a higher energy level to a lower energy level. Quantum numbers describe the energy level, orbital shape, orbital orientation, and spin state of an electron. The principle quantum number, n, describes the energy level an electron is in. For example the electrons in the 3d orbitals are in the 3rd energy level, represented by n = 3. 4. The most stable arrangement of electrons is one with the maximum number of unpaired electrons. 5. No 2 electrons in the same atom have the same set of four quantum numbers. 6. Electrons from the s orbital will sometimes be promoted to a higher energy level in order to form an electron configuration of lowest energy, which then is more stable. 7. The colors are created by photons containing specific amounts of energy, released when an electron makes the transition from a higher energy level to a lower energy level. 8. Ground-state electron configurations a. C 1s2 2s2 2p2 b. K 1s2 2s2 2p6 3s2 3p6 4s1 c. Ga 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p1 d. Cu 1s2 2s2 2p6 3s2 3p6 3d10 4s1 9. 1 × 1012 m 10. 3.3 × 10−19 J