Electron Configuration Review
advertisement

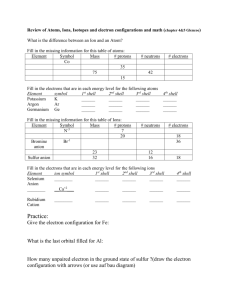
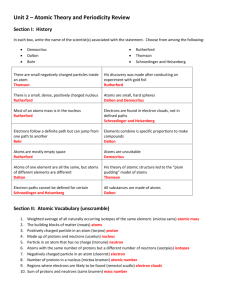
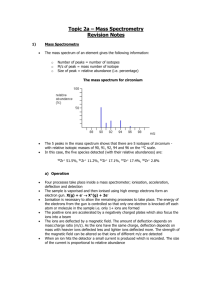
Electron Configuration Review Method FIRST, COLOR-CODE YOUR PERIODIC TABLE TO SHOW THE ORBITALS. Long Electron Configuration 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d10 1. The s orbital is ___sphere_____ shaped and can hold _2_ electrons. 2. The p orbital is ___dumbbell___ shaped and can hold _6_ electrons. 3. The d orbital is ___daisy___ shaped and can hold _10_ electrons. 4. The f orbital is ____funky___ shaped and can hold _14_ electrons. Short [Ar] 4s2, 3d10 Electron Configuration – Longhand Method NOTES Only the highest number of electrons present in each orbital (sub-level) is written in the electron configuration. Use the element symbol of the noble gas in the previous energy level enclosed in square bracket then append the remaining orbital notation. (The “last noble gas you pass”) Identify the following elements: Hint: if you add up all the exponents, it will equal the number of electrons. Also, if you look at the last number/letter combination, you can go directly to that spot on the periodic table to determine the element. Example: 1s2 2s2 2p6 3s2 3p6 4s2 3d3 (This has a total of 23 electrons which equals the atomic number of V – Vanadium.) Also, if you go to the third level of the d-block and count over 3 electrons you will see V. Both ways are good. Example: Electron configuration notations for Zinc, Zn [Z=30] Or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 [Ar] 4s2 3d10 When writing electron configurations, the total number of electrons must equal the exponents (longhand notation only) The electrons will fill the orbitals from left to right on the periodic table (in the same order as the atomic numbers) because it is arranged in order of lowest energy levels to highest energy levels. 1. _____Chlorine_____ 1s2 2s2 2p6 3s2 3p5 2. ______Oxygen___ 1s2 2s2 2p4 3. ______Cobalt___ 1s2 2s2 2p6 3s2 3p6 4s2 3d7 4. ___Strontium ___ 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 5. ____Silver _ 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d9 6. ______Selenium__ 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4 ______Cesium__ 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s1 7. Gold 8. 2 2 1s 2s 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d9 Write the electron configuration for the following elements using the longhand method. Then write it shorthand (Noble Gas) notation. Gold [Z=78] 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d8 7. Pauli exclusion principal states that every electron has a unique set of quantum numbers (electron configuration). The quantum numbers are EXCLUSIVELY unique to each electron. 8. Identify the following Elements: [Xe] 6s2 4f14 5d8 Silver [Z=47] 2 2 6 2 Carbon 6 2 10 6 2 9 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d Cromium [Kr] 5s2 4d9 Uranium [Z=92] 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2 5f4 [Rn] 7s2 5f4 9. Draw the orbital diagram for: (follow the diagram on #8 above) Hint: it may help to write the electron configuration first. Nitrogen [Z=7] Oxygen (Z=8) Which elements are represented by these noble gas configurations? [Ar] 4s2 3d6 Iron [Xe] 6s2 Barium [Ne] 3s2 3p1 Aluminum [Kr] 5s2 4d10 5p2 Tin . . . . Argon [Z=18] Another way to represent the electrons is called Orbital Notation. This is similar to the electron configuration, but it includes a diagram of the electrons represented by up and down arrows. The arrows indicate the opposite spins of the electrons within the orbitals. 5. The Aufbau Principle states that electrons will fill lower energy levels before filling higher energy levels. (Filling bottom/lower energy levels “off the bottom” sounds similar to Aufbau may help remember this name.) See textbook page 105 (and colored handout) 6. Hund’s Rule states that orbitals of equal energy are occupied by one electron (all three “p” orbitals will be filled with one electron first, then other electrons will share the “p” orbitals). Also, the spins will be parallel until the orbitals are shared. See textbook example page 106. a. 1s2 2s2 2p4 b. 1s2 2s2 2p6 3s2 3p6 4s2 3d 4 Potassium [Z=19]