Ionic vs. Molecular Compounds: Key Differences
advertisement

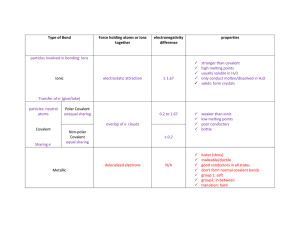
Ion: an electrically charged atom or group of atoms Molecule: a neutral group of atoms joined by covalent bonds Ionic Compounds Ionic bonds (between two oppositely charged ions) Form between metals and Molecular Compounds Covalent bonds (atoms share valence electrons) Form between two nonmetals nonmetals Ionic Bonds – very strong Covalent bonds – weak High melting point Low melting points and boiling points Conduct electricity when Poor conductors of electricity dissolved in H2O Form hard, brittle crystals Ex: NaCl (table salt) Ex: H2O C12H22O11 (sugar)