Oxidation Number Guidelines
advertisement

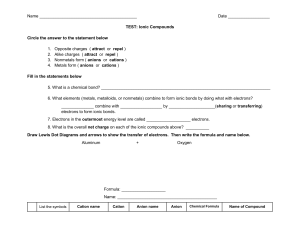
Oxidation Number Guidelines Oxidation number… 1. 2. 3. 1. 2. 3. 4. Of any uncombined element is 0. Of Group1 metals is always +1. Of Group 2 metals is always +2 Of Group 17 nonmetals is usually –1 (F is always –1) Of a monoatomic ions is equal to its charge Of H is usually +1 (except in metal hydrides, H is –1) Of O is usually –2 (except in peroxides, O is –1) For a neutral compound, sum of numbers must equal zero. For a polyatomic ion, sum of numbers equals the charge of ion. Oxidation Number Method for balancing redox reactions 1. Assign oxidation numbers 2. Identify what has been oxidized and reduced a. Place brackets b. Show gain and loss of electrons on brackets c. Multiply gain and loss of electrons by any subscripts d. Multiply by factors to make the gain and loss of electrons equal e. Add coefficients based on the multipliers on the brackets 3. Balance other atoms (except H and O) 4. If necessary, balance charges a. In acidic solution, add H+ where necessary b. In basic solution, add OH- where necessary 5. Balance O atoms by adding H2O where necessary 6. The H should check out.