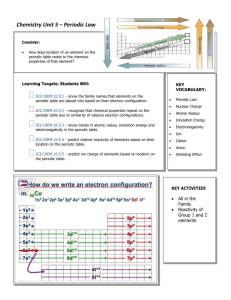
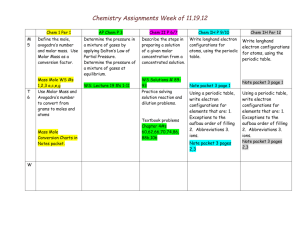
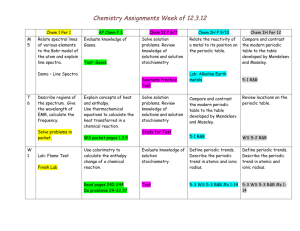
Chemistry Plans/Assignments Week of 12.10.12
advertisement
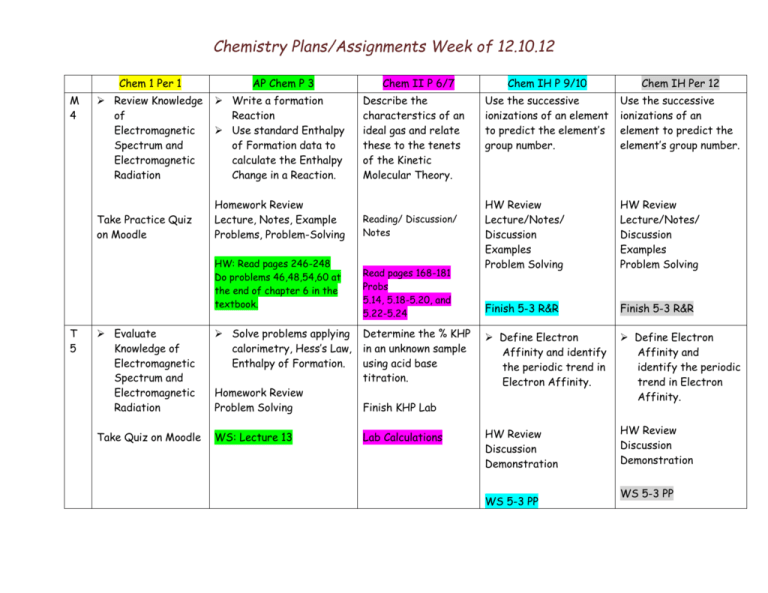
Chemistry Plans/Assignments Week of 12.10.12 Chem 1 Per 1 M 4 AP Chem P 3 Review Knowledge of Electromagnetic Spectrum and Electromagnetic Radiation Write a formation Reaction Use standard Enthalpy of Formation data to calculate the Enthalpy Change in a Reaction. Take Practice Quiz on Moodle Homework Review Lecture, Notes, Example Problems, Problem-Solving HW: Read pages 246-248 Do problems 46,48,54,60 at the end of chapter 6 in the textbook. T 5 Evaluate Knowledge of Electromagnetic Spectrum and Electromagnetic Radiation Solve problems applying calorimetry, Hess’s Law, Enthalpy of Formation. Take Quiz on Moodle WS: Lecture 13 Homework Review Problem Solving Chem II P 6/7 Describe the characterstics of an ideal gas and relate these to the tenets of the Kinetic Molecular Theory. Reading/ Discussion/ Notes Read pages 168-181 Probs 5.14, 5.18-5.20, and 5.22-5.24 Determine the % KHP in an unknown sample using acid base titration. Chem IH P 9/10 Chem IH Per 12 Use the successive ionizations of an element to predict the element’s group number. Use the successive ionizations of an element to predict the element’s group number. HW Review Lecture/Notes/ Discussion Examples Problem Solving HW Review Lecture/Notes/ Discussion Examples Problem Solving Finish 5-3 R&R Finish 5-3 R&R Define Electron Affinity and identify the periodic trend in Electron Affinity. Define Electron Affinity and identify the periodic trend in Electron Affinity. HW Review Discussion Demonstration HW Review Discussion Demonstration Finish KHP Lab Lab Calculations WS 5-3 PP WS 5-3 PP Chemistry Plans/Assignments Week of 12.10.12 W 6 In the atom: Relate the # of sublevels to the energy level State the # of e- that fill each energy level Describe the shape of spdf sublevels Notes, Discussion, Visuals Determine the Enthalpy Explain what of a Dissolution causes gas Reaction Using Constant pressure. Pressure Calorimetry. Convert between atm, mmHg, torr AP Chem Lab #6 : units of Calorimetry measurement. Explain how to HW: Lab Calcs and Qs read gas pressure from a manometer. Review knowledge of Periodic trends Review knowledge of Periodic trends HW Review Discussion- trend summary Review Worksheet HW Review Discussion – trend summary Review Worksheet Lecture/Notes/ Examples Problems 8-12, 26 TH 1 State the # of orbitals in an energy level and a sublevel. Describe the shape of spdf orbitals. Notes, Discussion,Visuals, Complete e- building charts. HW: EMR WS page 3 Examine the relationship between enthalpy, energy, and the first law of thermodynamics. Calculate the work done by a gas as it expands against a constant pressure. HW Review Lecture, Notes, Problem Solving Solve problems using moles, volume, temperature and pressure by applying the ideal gas law. HW Review Lecture/Notes Example Problems Problem Solving Read section 5.4 Problems Finish Review WS Review knowledge of Periodic trends Finish Review WS Review knowledge of Periodic trends HW Review Moodle Practice Quiz HW Review Moodle Practice Quiz Study For Test Study For Test Chemistry Plans/Assignments Week of 12.10.12 32,36,38,42,46,50 HW: Read pp 224-237 #s 11,14,16,18,20 in ch 6. F 2 Use a periodic Table to write long hand electron configurations for atoms. Notes, Discussion, Examples HW: WS Electron Configurations p.1 Week of 12.10.12 Examine the relationship between enthalpy, energy, and the first law of thermodynamics. Calculate the work done by a gas as it expands against a constant pressure. Read pp 224-237 #s 11,14,16,18,20 in ch 6. Solve stoichiometry problems using the ideal gas law Problem Solving Read Section 5-5 Problems 52-62 even. Evaluate knowledge of periodic trends. Evaluate knowledge of periodic trends. Moodle Test Moodle Test