Chemistry Unit 5 – Periodic Law
advertisement

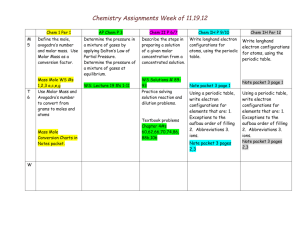
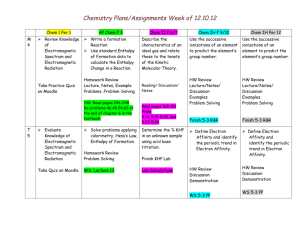
Chemistry Unit 5 – Periodic Law Electronegativ itgyElectronEl e Consider: How does location of an element on the periodic table relate to the chemical properties of that element? Learning Targets: Students Will: SCI.CHEM.10.5.1 – know the family names that elements on the periodic table are placed into based on their electron configuration. KEY VOCABULARY: Periodic Law SCI.CHEM.10.5.2 – recognize that chemical properties repeat on the periodic table due to similarity of valence electron configurations. Nuclear Charge Atomic Radius SCI.CHEM.10.5.3 – know trends in atomic radius, ionization energy and electronegativity in the periodic table. Ionization Energy Electronegativity Ion Cation Anion Shielding Effect SCI.CHEM.10.5.4 - predict relative reactivity of elements based on their location on the periodic table. SCI.CHEM.10.5.5 – predict ion charge of elements based on location on the periodic table. KEY ACTIVITIES All in the Family Reactivity of Group 1 and 2 elements