Learning Targets: Students Will: SCI.CHEM.10.4.1
advertisement

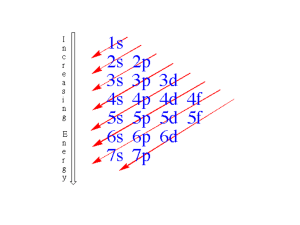
Chemistry Unit 4 – Atomic Structure Consider: Where does light come from How do the transition metals have multiple valences. Learning Targets: Students Will: SCI.CHEM.10.4.1 - Students will know shapes and abbreviations Filling order of electrons of the electron orbitals Predict possible isotopes of a given element SCI.CHEM.10.4.2 - Students will construct accurate electron configurations for elements and ions, using noble gas shorthand as appropriate. SCI.CHEM.10.4.3 - Students will understand the difference between electrons in a ground state and an excited state SCI.CHEM.10.4.4 Student will state that observed colors both emitted and reflected correspond do wavelengths of electromagnetic radiation SCI.CHEM.10.4.5 Student will describe the location, size, mass and charge of the sub-atomic particles Bohr Equation: -313.6 kcal/mol en2 KEY VOCABULARY: Proton Electron KEY ACTIVITIES Neutron Nucleus Energy Level Orbital Isotope Atomic number Atomic Mass On worksheets and a unit test be able to Predict possible isotope given an element Calculate the number of protons, electrons and neutrons given an element. Write electron configurations for elements, and ions. Calculate the energy absorbed or emitted from a specific electron transition. Isoelectric (isoelectronic) Electron configuration