Enthalpy: Definition, Formula, and Thermodynamic Derivation
advertisement

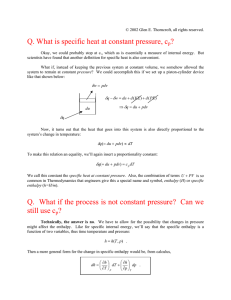
Enthalpy Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure. The enthalpy of a homogeneous system is defined as[5][6]: where H is the enthalpy of the system U is the internal energy of the system p is the pressure of the system V is the volume of the system. The enthalpy H(S,p) of homogeneous systems can be derived as a characteristic function of the entropy S and the pressure p as follows: we start from the first law of thermodynamics for closed systems for an infinitesimal process Here, δQ is a small amount of heat added to the system and δW a small amount of work performed by the system. In a homogeneous system only reversible processes can take place so the second law of thermodynamics gives δQ = TdS with T the absolute temperature of the system. Furthermore, if only pV work is done, δW = pdV. As a result Adding d(pV) to both sides of this expression gives or So