Chemistry Assignments Week of 12.3.12
advertisement
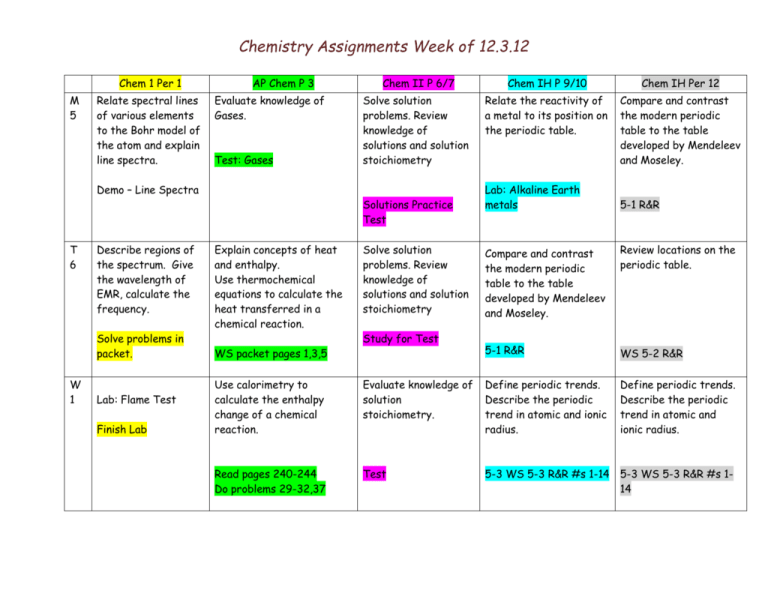
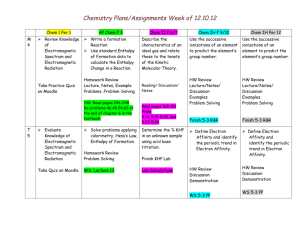
Chemistry Assignments Week of 12.3.12 Chem 1 Per 1 M 5 Relate spectral lines of various elements to the Bohr model of the atom and explain line spectra. AP Chem P 3 Evaluate knowledge of Gases. Test: Gases Demo – Line Spectra T 6 Describe regions of the spectrum. Give the wavelength of EMR, calculate the frequency. Solve problems in packet. W 1 Lab: Flame Test Finish Lab Chem II P 6/7 Chem IH P 9/10 Chem IH Per 12 Solve solution problems. Review knowledge of solutions and solution stoichiometry Relate the reactivity of a metal to its position on the periodic table. Compare and contrast the modern periodic table to the table developed by Mendeleev and Moseley. Solutions Practice Test Explain concepts of heat and enthalpy. Use thermochemical equations to calculate the heat transferred in a chemical reaction. WS packet pages 1,3,5 Solve solution problems. Review knowledge of solutions and solution stoichiometry Study for Test Lab: Alkaline Earth metals 5-1 R&R Compare and contrast the modern periodic table to the table developed by Mendeleev and Moseley. Review locations on the periodic table. 5-1 R&R WS 5-2 R&R Define periodic trends. Describe the periodic trend in atomic and ionic radius. Use calorimetry to calculate the enthalpy change of a chemical reaction. Evaluate knowledge of solution stoichiometry. Define periodic trends. Describe the periodic trend in atomic and ionic radius. Read pages 240-244 Do problems 29-32,37 Test 5-3 WS 5-3 R&R #s 1-14 5-3 WS 5-3 R&R #s 114 Chemistry Assignments Week of 12.3.12 TH 2 Solve problems using planck’s equation. Solve problems in packet. Draw enthalpy diagrams for endo and exothermic reactions. Use Hess’s Law of heat summation to calculate the enthalpy change of a chemical reaction. WS: Enthalpy diagrams WS: Hess’s Law F 3 Evaluate knowledge of EM spectrum. Practice Quiz/Quiz Use standard molar heats of formation to calculate enthalpy change in a reaction. Read pp 246-248 #s 39-42,46,47,49,51,52 , 54,58,60 Week of 12.3.12 Determine the % KHP in an unknown sample by acid base titration. Lab: Acid Base titration. Calculate the Molarity of your titrant. Determine the % KHP in an unknown sample by acid base titration. Lab: Acid Base titration. Finish Lab calculations. %KHP Define periodic trends. Describe the periodic trend in ionization energy Define periodic trends. Describe the periodic trend in ionization energy 5-3 WS 5-3 R&R #s 15-31 5-3 WS 5-3 R&R #s 15-31 Define periodic trends. Describe the periodic trend in electronegativity Define periodic trends. Describe the periodic trend in electronegativity 5-3 WS 5-3 R&R #s Finish 5-3 WS 5-3 R&R #s Finish