Chemistry Assignments Week of 11.27.12
advertisement

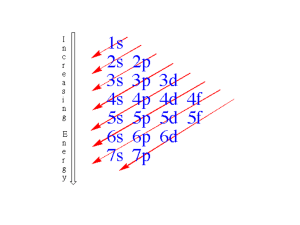
Chemistry Assignments Week of 11.27.12 Chem 1 Per 1 AP Chem P 3 Chem II P 6/7 Chem IH P 9/10 Chem IH Per 12 M No School No School No School No School No School T 1 Determine the identity of an element by measuring its molar mass in the lab. Review HW Lab: Molar Mass Describe the relationship between relative rates of effusion and diffusion of gases and the Molar Mass of the gas. Prepare a 0.1 M NaOH solution in preparation for KHP lab. Draw orbital diagrams for electron configurations of atoms and ions. Predict the magnetic behavior of elements using orbital diagrams. Draw orbital diagrams for electron configurations of atoms and ions. Predict the magnetic behavior of elements using orbital diagrams. WS: packet p.3 pages 4,5 WS: packet p.3 pages 4,5 Identify electron configurations of atoms in excited states. Identify electron configurations of atoms in excited states. WS: electron configuration review. WS: electron configuration review. Take Practice Quiz Evaluate knowledge of electron configurations. Take Practice Quiz Evaluate knowledge of electron configurations. W 2 Lab: Make 250 mL of 0.1M NaOH Finish Mole Lab Take Practice Quiz Lecture 20 #s 1-13 Evaluate knowledge ofMass Mole conversions. Determine the molar volume of a gas (oxygen) by collecting over water. Practice Quiz ,Quiz Lab Calcs. TH 3 Describe the relationship between wavelength and Review HW problems. Describe deviation from ideal gas behavior. Solve gravimetric analysis problems. Finish Alum lab calcs. (% sulfate) Read 148-150 ex.4.9 Problems 4.75-80 Define vocabulary relating to acid base titration. Solve acid Chemistry Assignments Week of 11.27.12 energy of light. Van Der Waals equations POGIL activity F 4 Explain how the electrons in an atom produce spectral lines. POGIL Activity Week of 11.27.12 base titration problems. Read 150-153 Ex 4.10,4.11 Practice Test, Test. Practice Test, Test. Lecture 19 # 14- end. Probs 105,133,136 Explain how to determine the molar mass of a gas by method of vapor density. Review knowledge of solutions and stoichiometry. Relate the reactivity of a metal to its position on the periodic table. Relate the reactivity of a metal to its position on the periodic table. Study for Test Monday Lab: Alkaline Earth metals Lab: Alkaline Earth metals Dry Lab – Molar Mass of Butane by Vapor Density Lab Calcs, Study for Test Monday.