Molecular Compounds WS
advertisement

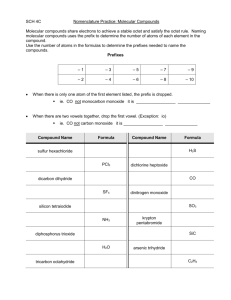
Name ___________________________________________ Date ____________ Period ______ Molecular Compounds Worksheet Molecular compounds are made of nonmetals atoms bonded together by sharing electrons. There are NO IONS and NO CHARGE involved in covalent bonding. When we name any molecular compound, we need to find the name of each element in the compound and to know the prefixes for the total number of atoms of each element in the compound. PART 1: Write each prefix next to the number that that it represents. 1 ____________ 2 ____________ 3 _____________ 4 ____________ 5 __________ 6 ____________ 7 ____________ 8 _____________ 9 ____________ 10 _________ PART 2: Name each molecular compound below. a) P3F4 _____________________________________________ b) Cl2O7 _____________________________________________ c) SO3 _____________________________________________ d) N2O5 _____________________________________________ e) CCl4 _____________________________________________ f) P2O5 _____________________________________________ g) SF6 _____________________________________________ h) NO _____________________________________________ i) N2H4 _____________________________________________ j) IF3 _____________________________________________ PART 3: Write the formula of each molecular compound. Tribromine nonoxide Dihydrogen monoxide Nitrogen dioxide Octanitrogen tetroxide Sulfur hexafluoride Phosphorus tribromide Carbon tetriodide Dinitrogen hexoxide Iodine monobromide Carbon monoxide