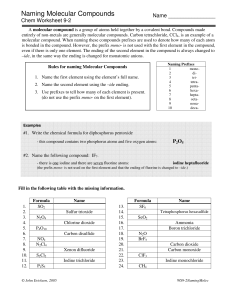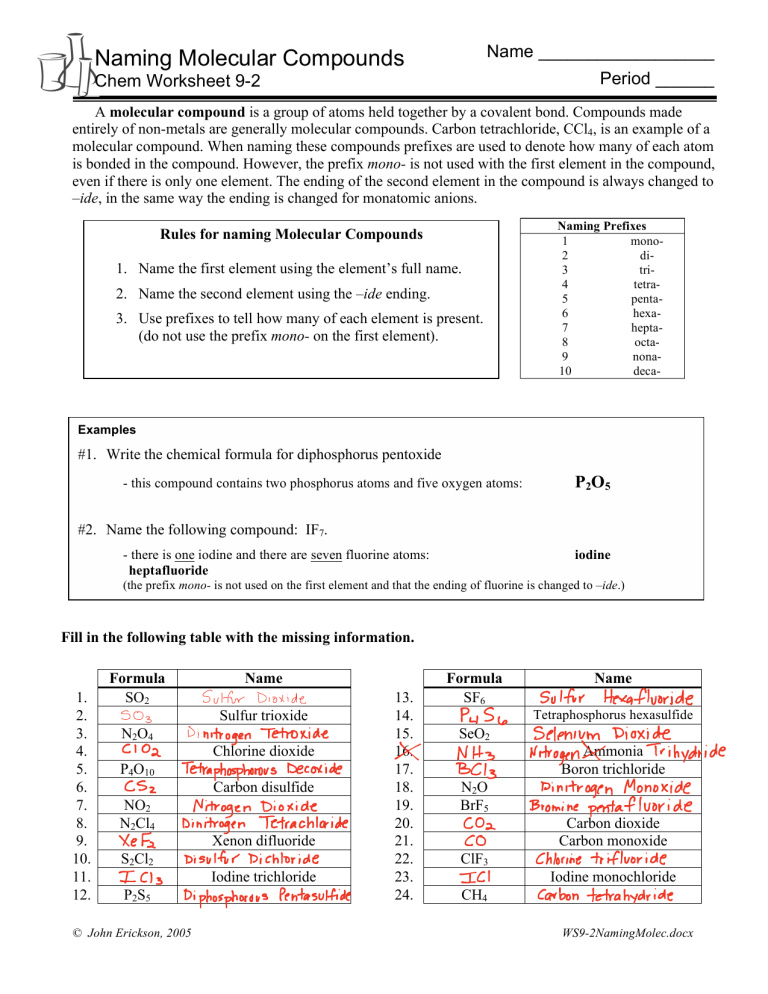
Name __________________ Naming Molecular Compounds Period ______ Chem Worksheet 9-2 A molecular compound is a group of atoms held together by a covalent bond. Compounds made entirely of non-metals are generally molecular compounds. Carbon tetrachloride, CCl4, is an example of a molecular compound. When naming these compounds prefixes are used to denote how many of each atom is bonded in the compound. However, the prefix mono- is not used with the first element in the compound, even if there is only one element. The ending of the second element in the compound is always changed to –ide, in the same way the ending is changed for monatomic anions. Rules for naming Molecular Compounds 1. Name the first element using the element’s full name. 2. Name the second element using the –ide ending. 3. Use prefixes to tell how many of each element is present. (do not use the prefix mono- on the first element). Naming Prefixes 1 mono2 di3 tri4 tetra5 penta6 hexa7 hepta8 octa9 nona10 deca- Examples #1. Write the chemical formula for diphosphorus pentoxide - this compound contains two phosphorus atoms and five oxygen atoms: P2O5 #2. Name the following compound: IF7. - there is one iodine and there are seven fluorine atoms: heptafluoride iodine (the prefix mono- is not used on the first element and that the ending of fluorine is changed to –ide.) Fill in the following table with the missing information. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. Formula SO2 503 N2 O4 102 P4O10 CSz NO2 N2Cl4 XeFa S Cl 2 ICI 2 P2 S5 Name SulfurDioxide Sulfur trioxide Dinitrogen Tetroxide Chlorine dioxide Tetraphosphorous Decoxide Carbon disulfide Nitrogen Dioxide Dinitrogen Tetrachloride Xenon difluoride DisulfurDichloride Iodine trichloride Diphosphorous Pentasulfide © John Erickson, 2005 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. X Formula SF6 R 56 SeO2 NHz Bcb N2 O BrF5 CO2 CO ClF3 ICI CH4 Name Sulfur Hexafluoride Tetraphosphorus hexasulfide Selenium Dioxide X Ammonia Trihydride Nitrogen Boron trichloride Dinitrogen Monoxide Bromine pentafluoride Carbon dioxide Carbon monoxide chlorinetrifluoride Iodine monochloride Carbontetra hydride WS9-2NamingMolec.docx