Naming the Aldehydes and Ketones
advertisement
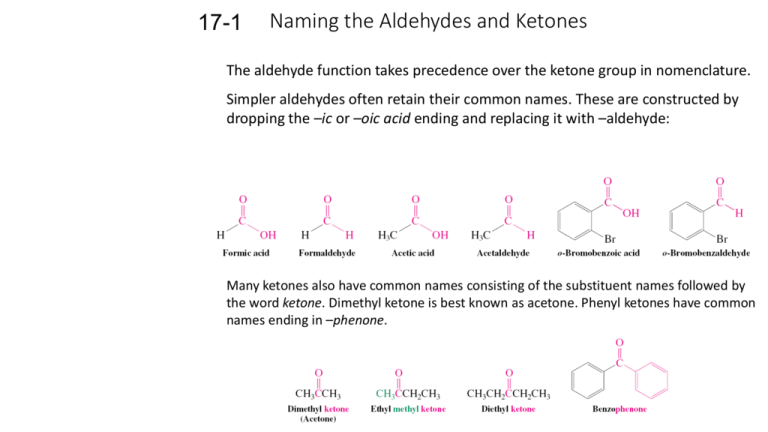
17-1 Naming the Aldehydes and Ketones The aldehyde function takes precedence over the ketone group in nomenclature. Simpler aldehydes often retain their common names. These are constructed by dropping the –ic or –oic acid ending and replacing it with –aldehyde: Many ketones also have common names consisting of the substituent names followed by the word ketone. Dimethyl ketone is best known as acetone. Phenyl ketones have common names ending in –phenone. IUPAC nomenclature treats aldehydes as derivatives of alkanes. The ending –e is replaced by –al. An alkane becomes an alkanal. Chem Abstracts retains the common names of the first two aldehydes: The names parallel those of the 1-alkanols except that the aldehyde carbon is assumed to be C1 and does not have to be specified. When an aldehyde is attached to a ring it is called a carbaldehyde. The carbon atom bearing the aldehyde is C1. The simplest aromatic aldehyde, benzenecarbaldehyde, is referred to using its common name, benzaldehyde, by Chem Abstracts. Ketones are called alkanones, the ending –e of the alkane is replaced with –one. IUPAC provides an exception for the simplest ketone, propanone, the common name of which is acetone. The carbonyl carbon of a ketone is assigned the smallest possible number, regardless of the presence of other substituents or the –OH, C=C or CC functional groups. Aromatic ketones are named as aryl-substituted alkanones. Ketones are called cycloalkanones when part of a ring. The carbonyl carbon is assigned C1 in this case. The systematic name of the fragment widely used. is “alkanoy.” The older term “acyl” is Both IUPAC and Chemical Abstracts retain the older names “formyl” and “acetyl” for the groups and . The term “oxo” denotes the location of a ketone carbonyl group when present with an aldehyde function. Several conventions can be used to denote the structure of an aldehyde or ketone: The condensed formula of an aldehyde must be written as RCHO and not RCOH, which might be confused with the hydroxy group of an alcohol. 17-2 Structure of the Carbonyl Group The carbonyl group contains a short, strong and very polar bond. The hybridization of the C and O atoms of the carbonyl group are sp2 hybridized. The C and O of the carbonyl and the two atoms attached to the carbon all lie in the same plane. The bond angles about the carbonyl are about 120o. The bond of the carbonyl is made of the remaining p orbital on the carbon and a similar p orbital on the oxygen. One of the simplest aldehyde is acetaldehyde: The primary electronic differences between a carbonyl group and an ordinary double bond are: •The oxygen contains two lone pairs in two sp2 orbitals; •The oxygen is more electronegative than carbon. The C=O bond is polarized; the carbon possesses a slight positive charge and the oxygen a slight negative charge. Polarization alters the physical constants of aldehydes and ketones. Due to the polarization of their carbonyl groups, the boiling points of aldehydes and ketones are higher than hydrocarbons of similar molecular weights. Acetaldehyde and acetone are completely miscible with water. As the hydrocarbon length increases, solubility decreases. Carbonyl compounds have more than six carbons and are relatively insoluble in water.









