Sc. 404: Ch.1 TB Solutions
advertisement

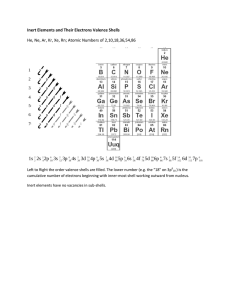
Sc. 404: Ch. 1 TB Solutions 1) An atom is too small to be seen. 2) b. a) Water because it appears uniform. Sand because the grains represent atoms. 3) No, because different elements have different masses and sizes. The hydrogen should be smaller than the oxygen atom. 4) - 6 OMIT 7) The proton 8) Atoms will gain energy, causing electrons to become excited. The electrons then jump to the next energy level. When they return back to their original shells, they release energy, emitting a specific colour light. 9) a) An atom is neutral b. Atom is mostly empty space c. Nucleus is small, dense and positive d. Electrons jump to a higher level of energy and release the energy in a form of light 10 - 12 OMIT 13) Right of the staircase, except hydrogen 14) They have the same number of valence electrons, so they have similar chemical properties. 15) Because hydrogen has only 1 valence e-, like all the alkali metals. 16) Alkaline Earth Metals. They have 2 valence e-. 17) D, because it is the electron on the last shell. 18) Same number of electron shells 19 - 27 OMIT 28) Lewis Notation only shows valence e29) a) Correct 30) b) Incorrect; Ar has 8 valence e- c) Correct 31) a) Correct c. No, P+ should equal to E- b) No, P+ should be in nucleus and E- should be on the shells d) No, P+ should equal to E-